Sterile Water For Injection: The Complete FAQ Guide In 2025
How sterility is ensured during the preparation of injection solutions? The injection formulations are hygienically made to avoid even a trace of contamination. It is pivotal for the health of patients because, without it, individuals will suffer from serious adverse reactions and in some cases fatalities.
For this purpose, pharmaceutical and healthcare sectors usually utilize sterile water for injection. Its purpose is to dilute intravenous drug injections. It is a pharmaceutical aid and sterile solvent for formulating injectable solutions. After adding a suitable solute, you can use it as a parenteral fluid replenisher.
You would be curious about the uses, benefits, pH range, endotoxin limits, packaging, and quality control testing of sterile water for injection. We have put together this FAQ guide to address your common queries and other associated topics about sterile water for injection. So, let’s delve deeper into this topic.
1.How to define sterile water for injection?
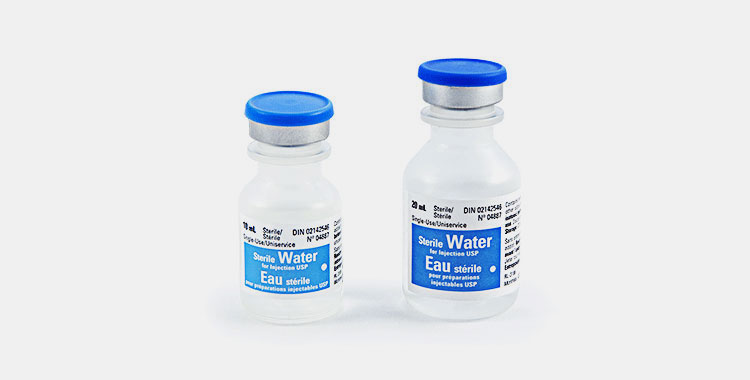
Sterile Water for Injection- Picture Courtesy: Dufort et Lavigne
It is sterile and pure water, free of different kinds of bacterial, fungal, and other microbial agents. It is produced by cleansing the potable water with the help of distillation and reverse osmosis. Different types of impurities, for instance, organic molecules, inorganic ions, endotoxins, pyrogens, nucleases, dust, and debris are removed from the purified water to make it fit for injection purposes.
Furthermore, it does not have any traces of bacteriostat, antimicrobial preservatives, or buffers. Sterile water for injection is typically employed in parenteral preparations and is utilized as a solvent for diluting the formulation. It is manufactured under sterile and aseptic conditions and is packed in single-dose containers to dissolve parenteral drugs.
2.What are the primary advantages of sterile water for injection?
Who wouldn't want to know the benefits of a product as crucial as sterile water for injection? Why it is routinely used in injection preparation? So, to ease your mind, here are some primary advantages of sterile water for injection:
Improved Purity
Improved Purity- Picture Courtesy: Farris Labs
As you know sterile water for injection does not have any kind of particulate matter or microbial presence; hence, it is part of daily preparation of sensitive medications. Besides this, there are no added buffers, salts, additives, or other compounds in sterile water for injection, which makes it safe for the reconstitution of a wide array of powdered, lyophilized, and freeze-dried chemical and biological medications.
High Sterility
High Sterility- Picture Courtesy: B.Braun
You would certainly have used sterile water for injection for formulating the intravenous, intramuscular, and subcutaneous injections. Why is that? Well, these preparations require a high degree of sterility because they are directly injected into the body without encountering stomach acidic environment that destroys harmful toxins and microbes.
This sterility is attained using sterile water for injection. The latter is prepared using stringent aseptic protocols that involve special measures to eliminate microbes from it. Henceforth, it averts the likelihood of sepsis or other infections.
Multitude of Use
Multitude of Uses- Picture Courtesy: Recipharm
The application of sterile water for injection is not limited to the reconstitution of medications. It is also utilized as a pharmaceutical support or solvent to make pharmaceutical solutions, suspensions, and, emulsions. Using this solvent, you can get precise drug dosing and appropriate solubility of hydrophilic drugs.
No Additives
No Additives
Sterile water for injection does not contain any type of electrolytes, as a result, it is hypotonic in nature. That’s why, pharmacists and chemists prefer to employ it for mixing a wide range of solutes and electrolytes and form isotonic solutions. Moreover, it is safely used for medicating infants and immune-comprised individuals because it is free of bacteriostatic agents.
Fulfills Regulatory Guidelines
Fulfills Regulatory Guidelines- Picture Courtesy: McGuff
You can ensure uniform quality across every product batch with the utility of sterile water for injection. Because it is prepared by following strict regulatory protocols and complies with USP, EP, and other global drug regulatory authorities. A standardized set of stages is employed for its preparation, thus, it is a universally renowned agent in healthcare organizations.
3.What are the uses of sterile water for injection?
Are you curious about the applications of sterile water for injection? It is utilized in the pharmaceutical, medical, diagnostic, and healthcare fields. Without delay, let’s discuss some significant uses of sterile water for injection:
Compounding in Hospitals
Compounding in Hospitals- Picture Courtesy: Fresenius Kabi USA
Medical specialists and pharmacists regularly use sterile water for injection for compounding tasks. It is a solvent that dilutes aseptic solution or suspension preparations. You can mix it with a wide array of substances, for instance, sodium chloride or dextrose to produce isotonic preparation for safe injection. It can be mixed with electrolytes for hydration therapy.
Diagnostic Use
Diagnostic Use- Picture Courtesy: SCHOTT Pharma
Today, you’ll find sterile water for injection in the diagnostic and research laboratories. To prepare contrast media and other diagnostic agents, sterile water for injection is utilized as a base to conduct imaging or tomography studies.
Medications for Children

Medications for Children- Picture Courtesy: Health-e
Yes, sterile water for injection is an ideal solvent for refashioning antibiotics, laxatives, and antacids. This is because sterile water for injection is not made with the addition of preservatives or bacteriostatic agents, making it safe for neonates and sensitive population groups.
Ophthalmic Use
Ophthalmic Use- Picture Courtesy: Marjan Yousefi
You would’ve used ophthalmic medications for curing eye infections and other disorders. How they are prepared? Since the eye is a sensitive organ, so ocular formulations are concocted using sterile water for injection. It is free of any microorganism or other chemical substances; hence you can safely utilize it to admix eye medications.
Veterinary Use

Veterinary Use- Picture Courtesy: Daily Paws
Sterile water for injection has also found its use in the veterinary industry. Certain vet medications are available in powdered or concentrated form. Sterile water for injection is utilized to dilute and dissolve these therapies.
4.How to prepare sterile water for injection?
Sterile water for injection is an extremely pure and sterile solvent, as, pharmaceutical manufacturers prepare it in a highly controlled environment to maintain its sterility. Now let’s learn about various steps for preparing sterile water for injection:
Water Purification

AIPAK ENGINEERING Multi-effect Water Distiller
In the first step, municipal water is passed through different extreme purification stages to clear various impurities, such as chlorine, contaminants, organic compounds, particulate matter, and other debris. Some frequent approaches to purify water are:
| Reverse Osmosis
|
The pretreated water is moved across a semi-permeable membrane to eliminate soluble salts and other small contaminants. |
| Deionization
|
How are ions removed from the pretreated water? A method called deionization is utilized for eliminating ions, like calcium, magnesium, or sodium from treated water. Ion-exchange resins with functional groups attract and capture ions in this process. |
| Ultrafiltration
|
It is a membrane filtration method in which water is forced with high pressure across the semipermeable membrane. It filters out the larger particles, for instance, bacteria, viruses, and larger molecules. The membrane has a pore size of 0.01 to 0.1 microns, which is ideal for filtering larger particulates. |
| Distillation
|
It is a highly popular and widely utilized method for preparing high-grade purified water. Water is boiled and then steam is condensed back to remove volatile and non-volatile compounds. It separates different dissolved compounds in the liquid depending on their boiling temperatures. |
Sterilization

UV Sterilization- Picture Courtesy: CleanLink
After filtration, the water is purified using various sterilization techniques, for instance:
| Steam Sterilization | It is the most important and widespread technique for sterilizing purified water using autoclave. In this approach, high-pressure hot steam is passed through water to kill various microbes and pathogens. |
| UV Sterilization | It is an additional sterilization step for killing heat-resistant microbes and endotoxins with the help of UV radiation. |
| Membrane Sterilization | In this sterilization technique, the specialized filter is utilized to eradicate microorganisms and pyrogens from the purified water. It retains bacteria, viruses, fungi, and algae on the filter membrane while purified water goes across it. |
Quality Control Testing

Quality Control Testing- Picture Courtesy: Bio-Rad
Various tests are conducted to ensure that the sterile water for injection satisfies the strictest criteria of regulatory compliance. Diverse investigations, for example, microbial, chemical, and endotoxin tests are performed to investigate the sterility and purity of sterile water for injection before filling.
Aseptic Filling and Packaging

Aseptic Filling- Picture Courtesy: BWT Pharma
Finally, sterile water for injection is dispensed inside the sterile containers and is sealed instantly to uphold the highest level of sterility. The filling and sealing of containers are carried out under aseptic conditions so that no viable microbial contaminant can enter the container.
5.What is the pH range of sterile water for injection?
Ph Range of Sterile Water For Injection
Maintaining the correct range of pH is pivotal, as numerous drug ingredients are pH-sensitive. So, any fluctuation in pH from the set threshold causes degradation of components. This decreases the efficacy of medications. Furthermore, unsuitable pH causes necrosis of tissue or irritation on the injection site. Moreover, If this pH deviates from the physiological limit, then it could destroy red blood cells.
Therefore, it is recommended to maintain the pH of sterile water for injection at 5.5 (5.0 to 7.0). This pH is near neutral but it is somewhat acidic due to the entrapment of carbon dioxide from the surrounding.
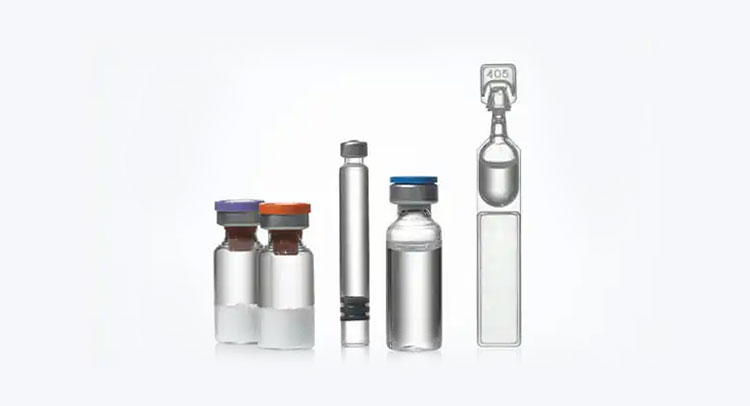
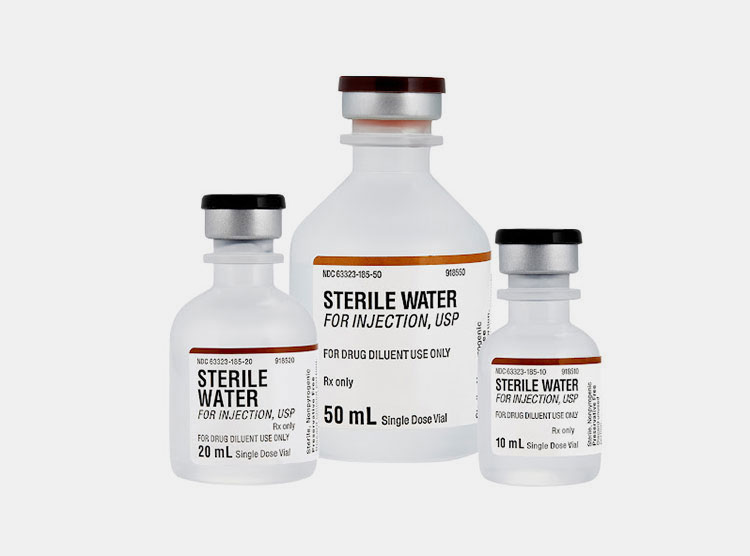
6.In what packaging sterile water for injection is available?
Since sterile water for injection is high in demand consequently, it is packed in diverse packaging formats to keep medication sterile. These packaging styles aid in offering ease of use and are suitable for different medical uses. So, let’s learn about the details of frequent packaging options:
Ampoules
Ampoules- Picture Courtesy: Tradeindia
These are the tiny glass or plastic containers that are melted and sealed by hot flames to completely barricade the entry of contaminants and air. They are broken to access the sterile water for injection. They are ideal for single use and are commonly found in hospitals or emergency kits.
Vials
Vials- Picture Courtesy: Pfizer
They are cylindrical and round containers normally manufactured from glass. They are sealed with rubber stoppers and crimped using aluminum caps. Precise amounts of sterile water for injection are taken out of the vials using syringes.
IV Bags
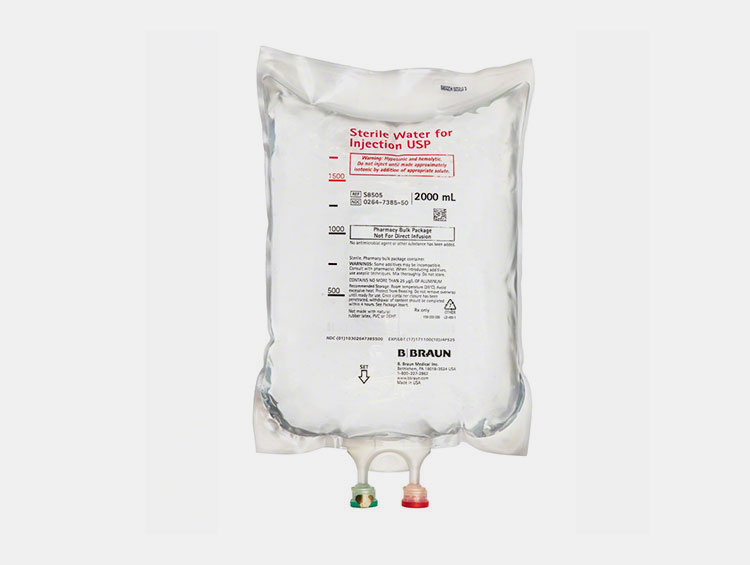
IV Bags- Picture Courtesy: B.Braun
These are flexible and pliable bags that store bulk volumes of sterile water for injection. They are typically created from PVC, PE, PP, or a mixture of PP and PE. They are commonly utilized in compounding IV solutions by mixing sterile water for injection with drugs or electrolytes.
Prefilled Syringes
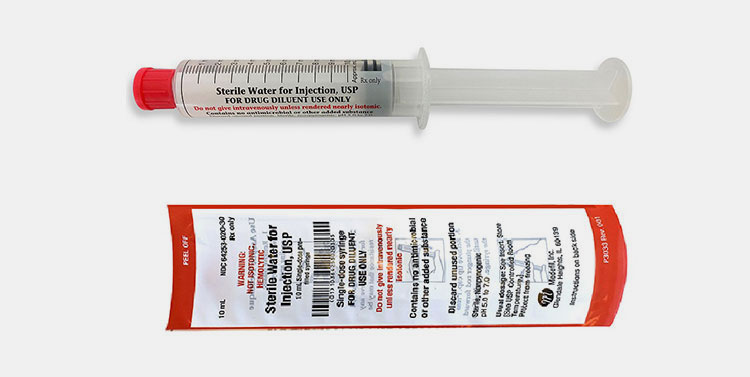
Prefilled Syringes- Picture Courtesy: Medefil Inc.
These containers are filled during production stages for direct use. It is easy to accurately reconstitute therapies using sterile water for injection syringes. Using these syringes, medical specialists avoid the risk of contamination and decrease preparation time.
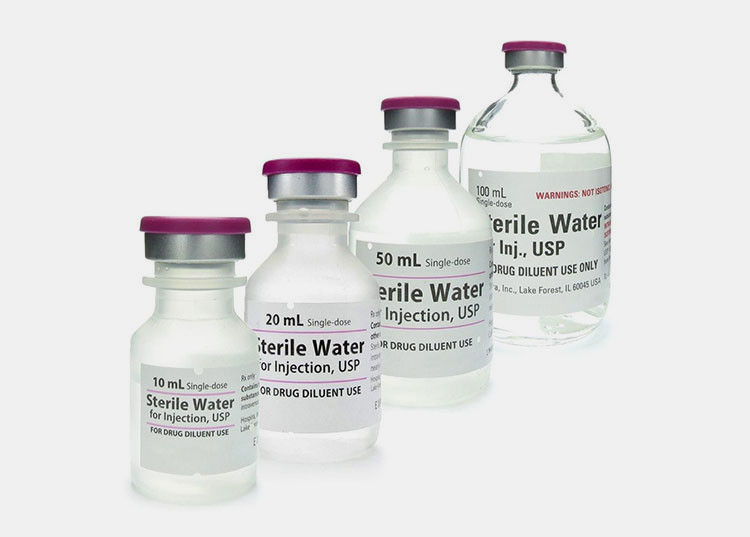
7.What are the available volume sizes for sterile water for injection?

Volume Sizes for Sterile Water for Injection- Picture Courtesy: ROVI Pharma Industrial Services®
You certainly would have seen diverse packing formats of sterile water for injection in the market. So depending upon the volume capacity of these packaging containers, there are different volume sizes of sterile water for injection.
The volume sizes for sterile water for injection go from 2 ml to as high as 3000 ml. For single-use (small volume) sterile water for injection containers, the standard sizes are 2, 5, 10, and 20 ml. As the name suggests, these volume sizes are good for single-time use only.
The bulk containers holding large volumes of sterile water for injection are about 1000, 2000, and 3000 ml.
8.Does sterile water for injection have an expiry date?
Expiry Date of Sterile Water for Injection- Picture Courtesy: McKesson Medical-Surgical
Are you wondering whether sterile water for injection is safe to use after a certain time? Absolutely, sterile water for injection is best for a certain period. You cannot use it beyond that time because it will lose its sterility and purity and become chemically unstable.
This expiry date is typically dependent upon the production methods and materials utilized in the packaging containers. The expiry date is present on the back of the sterile water for injection packaging and is around 2 to 5 years.
If the container is sealed and intact, then it will remain sterile over time. But it will lose its purity once the container is opened. Moreover, the increased storage will slightly alter the composition of sterile water for injection through the absorption of carbon dioxide.
Besides this, the packaging material may deteriorate with the passage of time, which leaches chemicals inside the sterile water for injection and jeopardizes the purity of the product.
9.How is sterile water for injection different from purified water and distilled water?
Sterile Water for Injection, Purified Water, and Distilled Water- Picture Courtesy: IV Fluids- Parenteral Solutions
Sterile water for injection, purified water, and distilled water all belong to the category of processed and filtered water. However, there are some slight variations between all of them. Some of the significant differences are stated below for your information:
| Feature | Sterile Water for Injection | Purified Water | Distilled Water |
| Description | It is a purified and sterile water used for reconstitution and injection purposes. It is devoid of any contaminant- whether physical or biological. | It is purified using different approaches, such as filtration to clear out harmful chemical or physical impurities, such as ions or debris, etc. | This processed water is purified using a distillation technique to eliminate various types of soluble solid, mineral, or chemical compounds. |
| Sterility | It is completely sterile. | It is non-sterile. | It requires further processing to attain a higher sterility level. |
| Pyrogens | It is completely free of pyrogens. | It contains pyrogens. | It also has trace quantities of pyrogens. |
| Degree of Purity | It is highly pure and fulfills every guideline of pharmacopeial regulations for intravenous, intramuscular, or subcutaneous injection. | It has high purity and is without any chemical, dust, sedimentary, suspended, or dissolved substances. | It is less pure than purified water and sterile water for injection. |
| Microbial Presence | There is no microbial contamination and populations of microbes are removed through thorough sterilization. | It has small amounts of microbes. | There is trace growth of microbes in distilled water. |
| pH | The pH of sterile water for injection is around 5 to 7. | It has a pH of about 7 but typically depends upon the purification method. | Distilled water also has a pH of 7 but it can capture carbon dioxide from air and turns slightly acidic. |
| Additives | None | No | No |
| Intended for Injection | It is intended for injection when it is admixed with electrolytes, solutes, and drugs. | No, it is not used for injection. | It is not employed for injection purposes. |
| Applications | Sterile water for injection is used for reconstitution and compounding. | Purified water is utilized in pharmaceutical production for cleaning pharmaceutical instruments. | It is used in research and diagnostic laboratories for preparing reagents and is also employed in instruments, such as humidifiers or steam presses. |
| Packaging Format | It is packed in ampoules, vials, and IV bags. | It is enclosed in non-sterile packaging formats, for instance, bottles or bulk containers. | Distilled water is also housed in non-sterile bottles and jerry cans or drums. |
| Regulatory Standards | It meets strict pharmacopeial regulations, such as USP, or EP. | It fulfills USP regulations for purified water. | It does not have any particular regulatory standard. |
10.What are the endotoxin limits for sterile water for injection?
Do you know what are the endotoxins? Well, they are fever-causing (pyrogens) substances that originate from the cell wall of gram-negative bacteria and produce various harmful and health-damaging reactions, for instance, fever when entered into the body. Consequently, the sterile water for injection should be free of endotoxins to prevent dangerous and life-threatening reactions in humans and animals.
According to the USP, the endotoxin limit should be less than 0.25 EU/ml in the sterile water for injection to avert the chances of infections and ensure patient safety. Maintaining this level of endotoxins boosts the product quality and increases the confidence of the public on brand reputation.
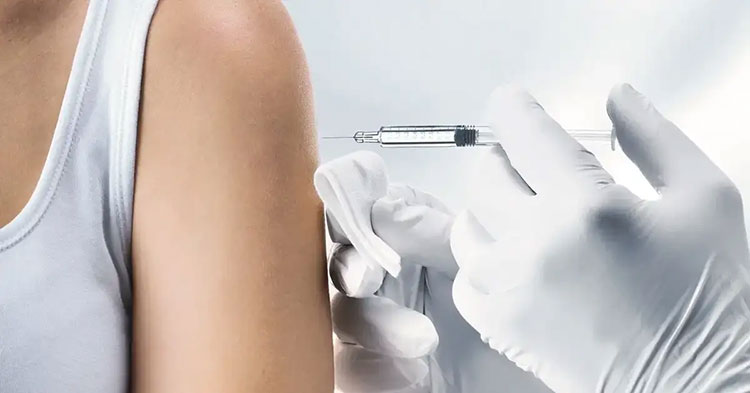
11.Can sterile water for injection be used for oral consumption?
Sterile Water for Injection- Picture Courtesy: Evoqua
This matter is commonly inquired. In short, the answer is no. You cannot use sterile water for injection for oral consumption or drinking purposes. It is not developed for the medical and pharmaceutical functions. Why is that?
First of all, it has low osmolarity and does not have any electrolytes or solutes so if it is drunk, then it could alter the electrolyte imbalance in the body, resulting in water intoxication. It is approved only for injection or dilution applications. Secondly, f it is used for oral consumption, then it could certainly infringe regulatory standards.
Last but not least, sterile water for injection is quite expensive, and less sensible to use it for drinking than purified water. You can consume portable, purified, or distilled water. However, in emergencies, you can drink only a small volume of sterile water for injection.
12.Can sterile water for injection be reused after opening the container?

Reusing of Sterile Water for Injection
No, you should not reuse the sterile water for injection after opening the container. It is only reused if it is labeled and created for multi-use. Sterility is lost when the container is opened. It boosts the chances of contamination, thus, sterile water for injection is rendered unsafe for use. You could possibly get microbial infection by reusing it because single-use containers do not have antimicrobial preservatives.
Some sterile water for injection containers are marketed for multi-use and incorporate microbial inhibitors. So, you can remove the product from them over a predefined period- typically lasting around 28 days after opening. However, it is advised to use a sterile suction instrument for withdrawing sterile water for injection from bulk-volume containers to retain its purity.
13.What are quality control tests for sterile water for injection?
Quality Control Tests for Sterile Water for Injection- Picture Courtesy: Pharmacist Digest
Pharmaceutical developers conduct rigorous quality control tests to ascertain that sterile water for injection is sterile, pure, and fit for consumption. These tests are performed according to guidelines outlined in the global pharmacopeial standards, for example, United States Pharmacopeia (USP) and European Pharmacopoeia (EP). Some important quality control tests for sterile water for injection are penned below:
| Test | Description |
| Sterility Test | This test is carried out to ensure that sterile water for injection is free of living pathogens. It is conducted using various types of culture media under an aseptic environment. If no microbe is detected, then it indicates the sterility of the product. |
| Endotoxin Test | It confirms that there is no pyrogen present in the product. A Limulus amebocyte lysate (LAL) test is performed to determine the level of endotoxins in the sterile water for injection. In this test, a reagent from the blood cells of the horseshoe crab is used for identifying endotoxins. When this reagent encounters endotoxins, it forms a visible clot, which shows the presence of even minute amounts of endotoxins. |
| pH Test | You can confirm the pH range of sterile water for injection using a calibrated pH meter. |
| Conductivity Test | In this test, ionic particles are quantified to confirm the absence of ions. The electrical conductivity of sterile water for injection is checked by a conductivity test and low conductivity is the measure of high purity. |
| Total Organic Carbon Test | To investigate the amount of organic compounds in the sterile water for injection, a total organic carbon test is conducted. The sample is loaded in the heated furnace and oxidation of organic carbon occurs in this furnace, which produces carbon dioxide. The quantity of the latter is detected by the infrared detector. |
| Appearance Test | In this procedure, the physical appearance and clarity of the sample are analyzed. Clear, odorless, and, non-cloudy sterile water for injection is the mark of sterility when checked against a white and black background. |
| Particulate Matter Test | This analysis confirms that sub-visible particles with sizes e.g., ≥10 µm, ≥25 µm are present within the specified limit. It is carried out using light obscuration or a microscope. |
Conclusion
Sterile water for injection is the go-to for reconstituting, compounding, and diluting the parenteral injection formulation and powdered medications in hospitals, pharmacies, research laboratories, and diagnostic centers. It is routinely utilized for its high purity and the fact that it does not have any microbial, pyrogen, ionic, organic, particulate, dust, or other type of contamination. It is ideal for guaranteeing the safety of patients. Hopefully, through this FAQ guide about sterile water for injection, you will come to know about utilities, benefits, quality tests, and more about this type of processed water. If there are more queries on your mind regarding sterile water for injection or if you require equipment to produce it, then you are more than welcome to contact AIPAK Engineering.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours

