Purified Water In Pharmaceutical Industry:The Complete FAQ Guide In 2025
Purified water is the process of removing impurities and ions from water through various water treatment techniques to obtain high-purity water quality. Purified water plays an important role in the pharmaceutical industry and has multiple uses.
However, purified water in the pharmaceutical industry is related to the quality of the final product and also to human life and health, so its testing needs to meet certain standards.
Do you know any treatment methods for purified water in pharmaceutical industry? And what are its uses? You must have many questions about purified water in pharmaceutical industry.
Next, let’s learn about the complete FAQ guide on purified water in pharmaceutical industry
1.What is Purified Water In Pharmaceutical Industry?

Purified Water In Pharmaceutical Industry - Sourced: smartwatermagazine
Purified water in pharmaceutical industry, in simple terms, is high-purity water obtained after a series of precision treatments. This type of water can be extracted through various methods. In addition to the pharmaceutical industry, it can also be used in biochemistry, chemical engineering, and hospitals.
Almost all impurities, metal ions, organic matter, and microorganisms have been removed in the purified water. The goal of purified water is to remove these impurities to meet the requirements of specific industries or applications.
2.What Uses Purified Water In Pharmaceutical Industryhas?
As is well known, purified water plays an important role in the pharmaceutical industry. Do you know what uses purified water has?
Production of topical medications

Production of topical medications - Sourced: Prescription Hope
Some oral and topical medications, such as ointments and cleaning solutions, cannot be directly prepared using regular tap water. As is well known, ordinary tap water contains many impurities and microorganisms, which are not suitable for pharmaceuticals. Therefore, purified water is required for production.
Production of pills

Production of Pills - Sourced: Soracom
In the production process of certain pills and capsules, purified water can also be used as an auxiliary raw material. The production of drugs cannot do without purified water, which can be used as a solvent for drugs and has functions such as dilution and dissolution.
Cleaning medical equipment
Cleaning Medical Equipment - Sourced: Medisafe Philippines
Purified water can also be used to clean medical instruments and surgical sites for the sake of sterile production equipment and environmental hygiene. Therefore, it can effectively remove some impurities and pollutants.
Source water for water of injection

Water of Injection - Sourced: Healthxchange Ireland
Purified water can be used as raw water for water of injection. Obtained by distilling and cooling purified water in a specially designed distiller, it is used for other pharmaceutical purposes.
3.What is the Difference between Purified Waterand Water for Injection in Pharmaceutical Industry?

Purified Water and Water for Injection - Sourced: www.cti-cert.com
Purified water and injection water are two common types of water in the pharmaceutical industry. What do you know about these two types of pharmaceutical water? Are you curious about their differences?
| Purified water | Water for injection | |
| Character | Colorless and odorless liquid. | Colorless liquid. |
| Standard | It must comply with USP and EP standards for endotoxins. | It also must comply with USP and EP standards for endotoxins, and it is higher than purified water. |
| Production | The production process includes methods such as reverse osmosis and deionization. | The production process must include distillation, dual channel reverse osmosis, and ultrafiltration. |
| Cost | The production process is relatively simple, so the cost is relatively low. | The production process is more complex and the quality standards are more stringent, resulting in higher costs. |
| Storage | Generally prepared before use and stored at room temperature. | It can be kept at 80℃ or above, kept at 65℃ or above for circulation, or stored at 4℃ or below. |
| Use
|
It can be used as a daily cleaner and preservative. | It is mainly used for diluting drugs and can also be used for flushing during surgery. |
4.How Does Purified Water In Pharmaceutical IndustryImpact Pharmaceutical Product Quality?
The quality of drugs is related to our life and health. Do you know how pharmaceutical purified water affects the quality of drugs? This involves purified water itself as well as production equipment and processes.
Quality of purified water

Quality of Purified Water - Sourced: SMAC gig WORLD
The water used in the pharmaceutical process comes into direct contact with drugs, and purified water is used in the production process of drugs, such as raw materials, solvents, etc., which are related to the quality of drugs. Therefore, the quality of water directly affects the quality of drugs.
It can effectively remove impurities such as microorganisms, organic matter, inorganic matter, and particulate matter from water, ensuring that the water used in the drug production process meets the standards specified in the pharmacopoeia, thereby ensuring the safety and effectiveness of the drug.
Quality of production equipment

Quality of Production Equipment - Sourced: Dimension Software
If the production equipment used does not meet the production standards or is contaminated, it will affect the quality of the final purified water, thereby affecting the quality of the final pharmaceutical product.
Once the equipment is contaminated, it may cause problems such as bacterial contamination and foreign object contamination of drugs, affecting the quality of drugs.
The production process

The Production Process - Sourced: www.toppr.com
Drug production must strictly comply with relevant regulations and standards to ensure the safety and effectiveness of drugs. Purified water, as a high standard process water, meets the high requirements for water quality in pharmaceutical production.
When using purified water to produce drugs, it is also necessary to comply with relevant regulations in order to pass the review of the drug regulatory department smoothly.
5.What is the USP Standards for Purified Water In Pharmaceutical Industry?
USP refers to the United States Pharmacopeia, and purified water in the pharmaceutical industry must strictly meet USP standards.

United States Pharmacopeia - Sourced: Quality Matters
In the USP, there are clear requirements for various testing indicators for purified water in the pharmaceutical industry, as follows.
| Testing item | Standard |
| TOC | ≤0.5mg/L |
| Conductivity | ≤1.3μS/cm (25℃) |
| Microorganism | ≤100 cfu/ml |
| pH | 5.0 to 7.0 |
| Chloride | Not deeper than the control solution |
| Sulfate | No turbidity is allowed to occur |
| Ammonia | Volume<50ml, 0.6mg/L;
Volume≥50ml, 0.3mg/L |
| Oxidizable Substances | Pass the USP test |
In addition, the raw water for purified water must be at least drinking water, which complies with the national basic drinking water regulations issued by the United States Environmental Protection Agency.
6.What is the TOC Limit for Purified Water In Pharmaceutical Industry?

TOC - Sourced: Basic Water Science
TOC, as an important water quality indicator for measuring the degree of organic matter pollution in water bodies, can comprehensively reflect the degree of organic matter pollution in water. To ensure the suitability of water, especially in the pharmaceutical industry, TOC testing is very strict.
The higher the TOC value, the higher the organic matter content in purified water. If the total organic carbon is controlled at a relatively low level, it means that the pollution of organic matter, microorganisms, and bacteria in water is in a well controlled state. The TOC limit for purified water in the pharmaceutical industry is 0.5mg/L.
7.What is the Microbial Limit for Purified Water In Pharmaceutical Industry?
Microbial Limit - Sourced: LinkedIn
In order to prevent excessive growth of microorganisms during the use of purified water and when it becomes a part of the product, which may cause quality problems, microbial limit control is necessary.
The purified water system requires regular disinfection and microbiological testing to ensure that the water quality at the point of use meets the corresponding microbiological quality requirements. It is recommended that the total bacterial count in purified water be ≤100 CFU/ml.
8.What are the Causes of Contaminationof Purified Water In Pharmaceutical Industry?
Pharmaceutical water pollution can have serious consequences. If unqualified purified water is used for pharmaceutical processing, it will affect the production of the entire drug and increase the risk of human infection. The main causes of pollution include:
Pipeline system issues

Pipeline System Issues - Sourced: IndiaMART
The pipeline system needs to use stainless steel material and undergo passivation treatment. One of the reasons why purified water in the pharmaceutical industry is contaminated is that it may not have undergone passivation treatment, resulting in oxidation and corrosion on the surface of stainless steel.
Poor design of water supply system
Water supply system design is one of the most fundamental procedures in water treatment. One major reason for pharmaceutical water pollution may be related to the design of the water supply system, which may lack reverse osmosis filters, deionization systems, etc.
Improper operation

Improper Operation - Sourced: LinkedIn
When preparing purified water or performing equipment maintenance, if operators do not follow strict operating procedures, such as not wearing gloves, not using sterile workstations, etc., it may also lead to microbial contamination and cause endotoxin exceeding standards.
9.What Qualities Does a Tank for Storing Purified Water In Pharmaceutical IndustryNeed?
In order to ensure the quality of the final drug production, the requirements for storing pharmaceutical purified water tanks are also very high,
Corrosion resistance

Corrosion Resistance - Sourced: Stainless Steel Products
Storage tanks are generally made of 304 or 316 stainless steel material. This material does not react chemically with air or water, and has good corrosion resistance, which can extend the service life of the material.
Durable
Choosing durable storage tanks requires consideration of the water usage environment and requirements, and selecting appropriate materials to ensure the quality and purity of the water. This can extend the service life, ensure long-term operation, and save the cost of replacing storage tanks.
Having testing and inspection facilities

Temperature Sensors - Sourced: profilab24.com
Temperature sensors and other detection devices need to be installed in the storage tank. The temperature sensor can monitor the water temperature in real time and transmit the data to the control system. When the water temperature exceeds the set range, the control system will promptly issue an alarm to remind the operator to take corresponding measures to ensure the stability of the water temperature and the quality of the injection water.
Good sealing performance
The sealing of the storage tank is also very important and must be able to completely seal to prevent external pollutants from entering the water. In addition, the inner surface of the storage tank must be smooth, without any bumps or burrs, to prevent pollutants from adhering to the surface.
10.What is the Production Process of Purified Water In Pharmaceutical Industry?
To obtain pharmaceutical purified water, a series of production processes are required. Do you know which processes are involved?
(1)Pre-treatment
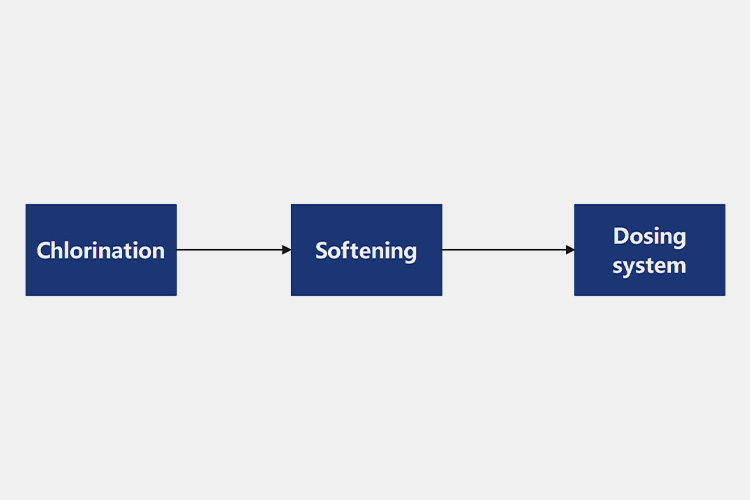
Pre-Treatment Process of Purified Water
Chlorination
The source water will contain a lot of suspended solids and other impurities, which need to be removed. When the organic content in the raw water is high, chlorination can be used for treatment. If it still cannot meet the inlet requirements of subsequent processes, measures such as activated carbon filtration can be added to remove organic matter.
Softening
When the hardness of carbonate in raw water is high, lime can be added to soften it while removing turbidity, turning hard water into soft water.
Dosing system
To maintain the purity of water, disinfection is necessary. Acid and alkali have bactericidal effects on microorganisms in purified water systems, effectively eliminating pollutants inside the system and ensuring stable and pure water quality.
(2)Post-treatment
Distillation, ion exchange or reverse osmosis
When the source water undergoes pretreatment, some obvious impurities can be removed, and then it enters the post-treatment stage. According to the factory’s production capacity and user needs, there are various methods for producing purified water, including distillation, ion exchange, and reverse osmosis. The specific methods are introduced in the following text.
Storage and pipeline transportation

Storage and Pipeline Transportation
After the above processing, the purified water will be directly stored in the water tank equipped with UV lamps to ensure the long-term stability of its water quality. According to different production site requirements, corresponding pipeline transportation facilities can be selected to meet the various usage needs of purified water.
11.What Treatment Methods of Purified Water In Pharmaceutical Industry?
There are various methods for treating purified water in pharmaceutical industry, each with its own advantages and disadvantages. The specific methods are as follows.
Distillation
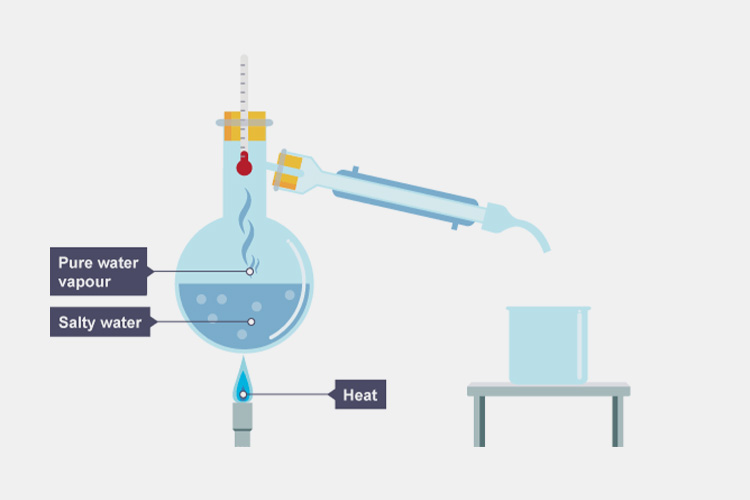
Distillation - Sourced: BBC
Distillation is the process of heating and evaporating water, and then cooling it to form a liquid. After multiple distillations, many impurities in the water, such as particles and bacteria, have been removed. This method consumes a lot of energy, wastes a lot of resources, and it is difficult to eliminate the dissolution of carbon dioxide, which can only meet the water needs of ordinary pharmaceutical factories.
Ion exchange
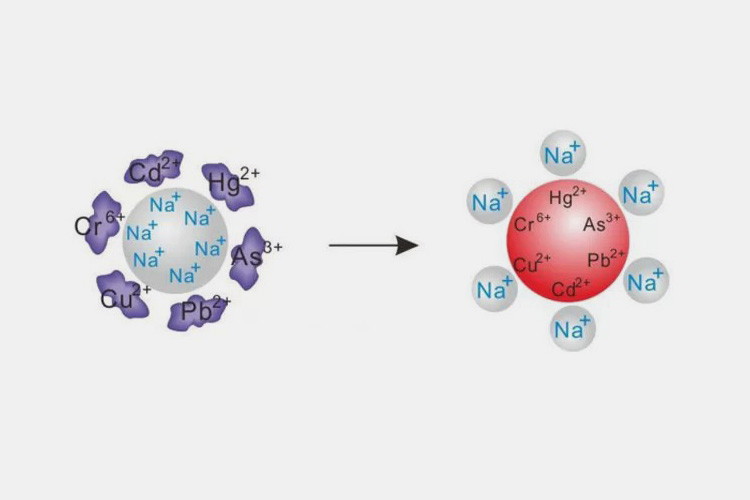
Ion Exchange - Sourced: Sunresin
Water is a mixture of many different atoms and molecules, containing many harmful substances. The method of purifying water by exchanging ions between anion and cation resins in order to extract purified water. This method can remove heavy metal ions and other organic compounds from water. Before conducting ion exchange, the water needs to be pre treated to remove suspended solids and other impurities.
Reverse osmosis
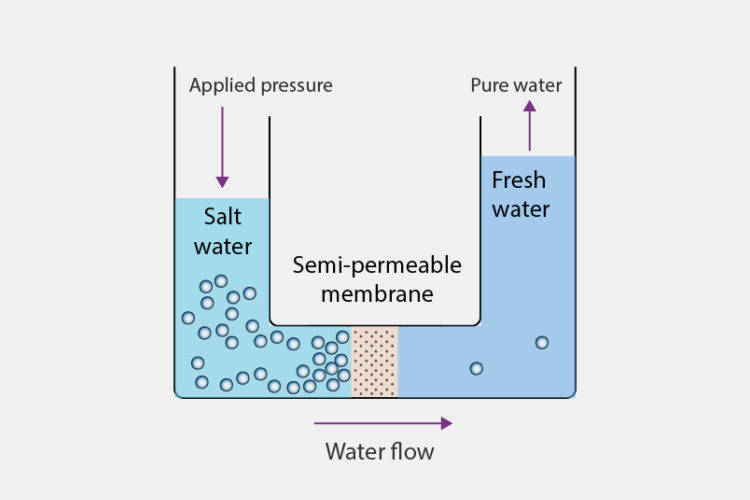
Reverse Osmosis - Sourced: BYJU’S
The use of reverse osmosis requires the first step of raw water pre-treatment to remove suspended solids and other impurities. Reverse osmosis refers to the process of removing dissolved organic matter, inorganic salts, plasma, and microorganisms from raw water through a semi permeable membrane under external pressure, resulting in relatively pure water.
UV irradiation
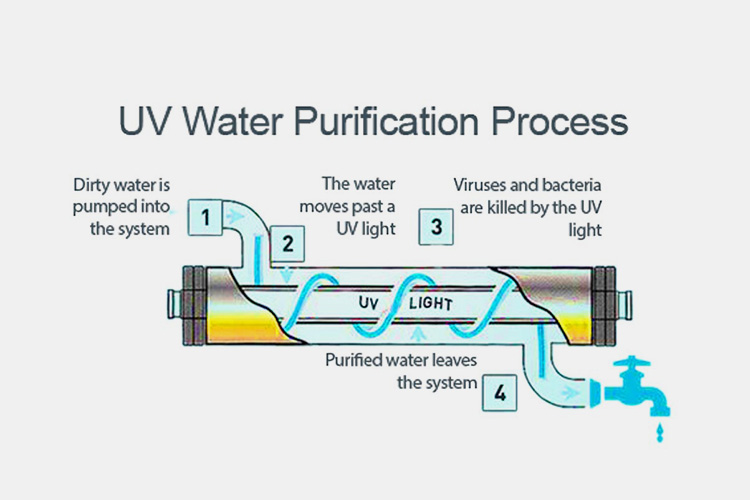
UV Irradiation - Sourced: Groundwater World
The ultraviolet irradiation method has been widely used in water treatment. The wavelengths for ultraviolet disinfection are 254nm and 185nm. This method can reduce the rate of new bacterial colony formation in the water system. Through irradiation, it kills no more than 90% of microorganisms in the water, as the DNA and proteins in bacteria absorb ultraviolet light and cause death.
Electrodialysis
This is a technology that uses ion selective permeation membranes under the action of an electric field for separation and purification. In the process of electrodialysis, the separated water molecules and ions migrate towards the positive and negative electrodes respectively, and the ions are intercepted on the ion selective permeable membrane, thereby achieving water purification.
12.What are the Requirements for the Installations of Purified WaterSystem In Pharmaceutical Industry?
The installation of pharmaceutical purified water systems needs to meet certain requirements. Specifically, as follows:
Requirements for pharmaceutical purified water

Requirements for Pharmaceutical Purified Water - Sourced: CPT Labs
According to the pharmacopoeia, purified water in pharmaceutical industry must meet a series of indicators, such as microbial limit requirements, to ensure its quality and safety. And the PH value of purified water needs to be within a certain range of 5.0-7.5.
In addition, pharmaceutical purified water needs to meet the requirements of cGMP, which is the legal standard for food, nutritional supplements, and pharmaceutical production operations. The document states that contact with drug ingredients and process materials should not react with the materials. Therefore, pharmaceutical purified water needs to meet this standard.
Conducting acceptance testing

Acceptance Testing - Sourced: SLA Institute
After purchasing the product, FAT and SAT are required, with the former being conducted at the manufacturer’s factory and the latter at the customer’s factory. Through these two tests, it can be ensured that the product has undergone quality testing and can understand the actual operation of the purified water system in the pharmaceutical industry.
Installation environment requirements

Installation Environment Requirements - Sourced: Wipro Lighting
Purified water equipment in pharmaceutical industry should be placed in a dry, ventilated, dust-free, non corrosive gas and vibration interference free environment, avoiding direct sunlight. Moreover, there should be no other equipment or items mixed around the equipment installation area, and it is necessary to ensure air circulation around the equipment to avoid pollution.
Installation location requirements
In order to maintain and operate easily, purified water equipment should be placed on a stable ground and kept at a certain distance from other equipment for easy maintenance and operation. At the same time, the placement of the equipment should be convenient for connecting water, electricity, gas and other supply pipelines, and the connections of the pipelines should be firm, leak proof and leak proof.
13.Why Choosing AIPAK EngineeringPurified Water Treatment System?
To remove impurities, harmful substances, and microorganisms from raw water and ensure that the final water quality meets high standards, AIPAK Engineering purified water treatment system is a good choice.

AIPAK Engineering Purified Water Treatment System
Optional RO and EDI
This device can be equipped with RO and EDI, which can shoulder the responsibility of desalination and purification of water quality. EDI has a deep desalination effect, which can obtain purer water quality than RO produced water. If higher quality purified water needs to be prepared, the RO+EDI mode is generally used, and EDI is placed behind the RO device.
In order to further ensure water quality safety, the EDI system is also equipped with ultraviolet sterilization and membrane filters to ensure that the produced water is always pure. In addition, the equipment is equipped with various safety devices, such as waterless protection and pressure protection, to ensure the safe operation of the equipment in all aspects.
PLC automatic control system

PLC Automatic Control System - Sourced: Motion Automation
This technology has high stability, anti-interference ability, and automatic error correction and exception handling functions. This system is easy to operate and convenient to install. In addition, this device adopts a touch screen, which can adjust parameters and is convenient to use.
Using a dedicated water tank

Using a Dedicated Water Tank
This device uses a dedicated water tank and is equipped with a pressure type liquid level gauge, as well as a rotating spray cleaning and air breathing device to ensure that the water tank is clean and pollution-free. In addition, the device is also equipped with ozone sterilization function, ensuring long-lasting high-quality water.
Conclusion
Purified water is an indispensable part of the pharmaceutical industry, and its importance is self-evident. This article takes you through a series of knowledge such as the uses and preparation methods of purified water in pharmaceutical industry, and you must have a certain understanding of purified water. Pharmaceutical water equipment is becoming increasingly advanced. If you have any needs or questions, please feel free to contact AIPAK Engineering at any time.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours

