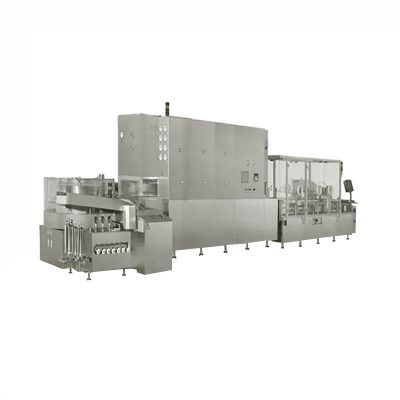
Vial Lyophilization Powder Injectable Powder Production Line
AIPAK Engineering vial lyophilization powder injectable powder production line is composed of ultrasonic bottle washing machine, sterilization dryer, filling and stoppering machine, capping machine. It can complete spraying water, ultrasonic cleaning, flushing of inner and outer wall of bottle, preheating, drying and sterilization, removing heat source, cooling, bottle clear-up, (pre-filling nitrogen), filling, (nitrogen filling), plug clear-up, press plug, cap clear-up, capping and other complex functions, realizing automatic production of the whole process. Each machine can be used separately, also made up production line, which is mainly used in production of vial injection and freeze-dried powder injection in pharmaceutical factory, can be applied to production of antibiotics, bio-pharmaceuticals, chemical pharmaceuticals, blood products etc.
Lyophilization powder example:
vial lyophilization powder injectable powder production line Features:
1.Outer feature: outer material is stainless steel, dull polished. The whole line is good in appearance, easy to clean. It complies with gmp regulations and can be suitable for several bottle specs.
2.Structure feature: water filling→ultrasonic wave coarsely washing→circulation water→circulation water + spraying WFI→compressed air→WFI→compressed air + spraying compressed air→tunnel oven preheating→high temperature sterilization→cooling→bottle feeding→filling→stoppering→cap-adding→cap-sealing→labeling→to the next process Filling dosage feature: no powder outside, filling error is small.
3.Running feature: stable running, low noise, precise filling, low energy consumption, low bottle breakage ratio, high qualified product ratio. The whole line can single machine control or interlock control. Washing machine 4 water and 4 air ensure washing effect; tunnel oven high temperature ensure removing pyrogen; servo motor ensure filling precision;10-head capping machine ensure sealing effect; labeling machine pneumatic coding ensure labeling speed.
4.Comply with gmp requirements. Contact part is made of SUS 316L, silicone rubber or ptfe material which is easy to clean and without contamination.
vial lyophilization powder injectable powder production line Technical Specification:
| Model | Production line | Suitable size | Output(max) | Power | Net weight | Overall size |
| APE I | CLQ 40 | 2.25 ml | 6000-12000 pcs/h | 69.8 KW | 7500 Kg | 9930×2500×2340 mm |
| RSM 620/44 | ||||||
| KGF 8 | ||||||
| APE II | CLQ 60 | 2.25 ml | 8000-18000 pcs/h | 85.8 KW | 8000 Kg | 10830×2500×2340 mm |
| RSM 620/60 | ||||||
| KGF10 | ||||||
| APE III | CLQ 80 | 2.25 ml | 10000-24000 pcs/h | 123.8 KW | 8100 Kg | 10830×2500×2340 mm |
vial lyophilization powder injectable powder production line Video
The Buyer's Guide
Lyophilized Powder for Injection: The Complete FAQ Guide In 2025
Nowadays, lyophilized powder for injection is considered an extensive mode of drug administration than other routes (oral) for bedridden individuals.
Picture Courtesy: BioAvenir
This is a significant route providing better compliance and a good stability profile. But what exactly is a lyophilized powder for injection? Why do you need it? There are many general questions that everyone has in their minds about products formed by the way of lyophilization.
In Lyophilized Powder for Injection: The Complete FAQ Guide In 2025, we have highlighted a detailed concept of lyophilized powder for injection and all related issues.
1.What Is Lyophilized Powder for Injection?

Lyophilized powder for Injection- Picture Courtesy: GEA
Over the past years, the pharmaceutical manufacturing industry has evolved with marked advancement in the development of new drug formulations. A lyophilized powder for injection is one of them. It is commonly known as a freeze-dried drug which is showing increased market demand due to improving the quality and shelf life of the potential active ingredients.
Lyophilization is simply a process of removing water content in the formulation and improving its stability. The process takes place by a few basic steps (we have discussed below) to produce a drug without the need for preservatives or unwanted additives, thus, making it more stable and offering long-term storage.
2.Why Prepare a Lyophilized Powder for Injection?

Lyophilized powder for injection
Conventionally, injection solutions were prepared in pharmaceutical formulations but why a new way of drug formulation has emerged via complex procedures? To answer this question, we have explained the following points.
Reason 1: Increased Shelf Life
A lyophilized powder for injection is a type of formulation where the proportion of moisture or water content is almost zero. Hence, this property ultimately stabilizes the trait of the following active ingredients which are present in powder form, and ensures that the drug is stable in long-term storage till you reconstitute them.
Reason 2: Safest Way of Preservation
You all know that drugs' high and safe life is mainly controlled by the addition of preservatives with limited concentrations. Indeed, preservatives are not considered a good option for many customers and physicians too as they’re hooked to some unwanted effects. However, using a lyophilized powder for injection is free from preservatives. This form of drug is made up by eliminating moisture and is useful in medical treatments.
Reason 3: Easy Storage
Whether vaccines or proteins, a lyophilized powder for injection does not require special temperature requirements or environments. You can store your product easily and transport it too; without the need for refrigerator or cold storage boxes. This is a convenient way to provide drugs to clinical setups with flexible storage options.
Reason 4: Better Solubility & Handling
The lyophilized powder for injection is estimated to be easily reconstituted when it comes to solubilized by water or other solvents which eventually ensures easy handling and dose management as compared to other forms of drug dosage form.
Reason 5: Reduced Risk of Contamination
Lyophilized powder for injection is prepared in a way that can maintain the sterility of the active product. This is pretty sure that freeze-dried products are known to be better dosage form where the invasion of microbes or other factors are almost negligible. Therefore, the chances for progression of contamination are very low and keep injectables stable.
Reason 6: Convenience for You and Physicians
Whether you’re taking protein or any hormonal therapies, lyophilized powder for injection is very convenient for you as it is more potent than other formulations. Similarly, physicians or healthcare providers especially from those areas where controlled conditions are improper, mostly prefer lyophilized powder for injection is more reliable to use.
Reason 7: Better Distribution & Economical
If you see the vials or containers for lyophilized powder for injection, it stores more potent powder acquiring a lesser space. Moreover, it is easier to distribute than liquid or other formulations. So, lesser storage, transportation, or distribution cost.
3.Where do you use Lyophilized Powder for Injection?
The industrial applications of lyophilized powder for injection are numerous. Some of
the major uses are mentioned below:
Health Care and Pharmaceutical Setups
Chemotherapy powder vial- Picture Courtesy: CTV News
To extend the quality, potency, and shelf life of the drugs, a lyophilized powder for injection is used in pharmaceutical preparations, such as antibiotics, chemotherapeutic drugs, protein therapy, temperature-sensitive medicines, enzymes, growth hormones, energy supplements, etc.,
Clinical Research and Development

Research and development- Picture Courtesy: Very well health
You can not use raw ingredients or test substances for clinical testing. It just has to be lyophilized first for proper consistency, solubility, and manageable handling. Thus, a lyophilized powder for injection is useful in testing and clinical trials.
Veterinary Medicine
Vet Lyophilized powder for injection
Just like humans, animals also need care and love! A lyophilized powder for injection is used for vaccination, supplements, antibiotics, and enzyme therapies for your pet. In this form, your pet is dealt with safe and effective medicines for the treatment of their ailments.
Nutraceutical Industry

Nutraceutical Supplements- Picture Courtesy: Verve cosmetics
The most popular and demandable manufacturing industry where day-by-day nutraceutical formulations such as probiotics, health supplements, and enzymes are prepared and packed in the form of lyophilized powder for injection.
Chemical Industry

Chemical Industry- Picture Courtesy: Vacuubrand
Various chemicals for testing and storing are prepared in lyophilized powder for injection formulation that is required for research testing and general assays.
4.What are the Vehicles for Diluting Lyophilized Powder For Injection?
To dilute lyophilized powder for injection, there are certain solvents or vehicles that form a potent solution for patient administration. The commonly used vehicles are described below:
Water for Injection (WFI)
WFI- Picture Courtesy: Technical Safety Service
The first thing that comes to mind when diluting lyophilized powder for injection is water for injection. This is sterile water that has no particular reactive substance in it such as additives or preservatives and is considered as the most suitable way of mixing when subjected to intravenous, intramuscular, or other route of administration.
Normal Saline

Normal Saline- Picture Courtesy: Medshop Australia
This is the most commonly used crystalloid globally in all clinical settings and is considered ideal for maintaining hydration. The normal saline doesn’t affect the potency of lyophilized powder for injection and is suitable for adults and children. This is available in various concentrations such as 0.9%, 0.45%, 0.33%, 0.18%.
Dextrose Solutions

Dextrose Solutions- Picture Courtesy: Promea
This is another vehicle commonly used when it comes to administering lyophilized powder for injection. This vehicle is almost safe to provide medicine except in diabetic patients where you need to monitor or avoid it as it may fluctuate the carbohydrate content in your body. For example, 5% dextrose is present in water or it is also known as D5W.
Ringer's Solution
Ringer Lactate- Picture Courtesy: Promea
You can also call it a lactated ringer solution which is an enriched electrolyte solution mostly used as a vehicle in patients to maintain electrolyte imbalances and used as an intravenous route for medication delivery. For example, patients of burn who suffer intense bodily physiological fluids, wound patients, or injuries.
5.How to reconstitute Lyophilized Powder for Injection?
Making a lyophilized powder for injection- Picture Courtesy: Empower pharmacy
To reconstitute lyophilized powder for injection, one must follow hygiene practices with proper presence of mind. Mentioned below are the main points when reconstituting the powder.
Before initiating the volume makeup, assemble the necessary supplies on a clear surface, wear the gloves, and push the cap over the vial of lyophilized powder.
Take a syringe and push the plunger to suck the water of injection or any vehicle gently and insert the needle of the filled vehicle inside the cork of the vial to add water into it. Once you ensure that the vehicle is drawn completely now take out the needle.
Gently tap the vial and turn it up and down to solubilize the medication completely. Once the medication is dissolved completely, take out the solution with the help of a needle taking care that the air bubble is not contained inside. If yes, then you should expel before administering to the patient by pushing the plunger and focusing the tip of the needle and air bubble expulsion.
6.What are Route of Administering Lyophilized Powder for Injection?
A lyophilized powder for injection can be taken by parenteral route soon after reconstitution with sterile water or other liquid vehicle. To administer you can use the following route of delivery medication.
Intravenous Lyophilized powder for Injection
| This is referring the delivery of medication by directly administering it into the vein mostly the hand or arm. The lyophilized powder for injection is either given as a bolus dose or slow infusion via line bypassing the need of 1st pass metabolism. This is a very important point to know as your drug can reach into blood before passing into a particular location such as the lungs, digestive tract, or liver, thus showing more bioavailability and rapid desired effects. | 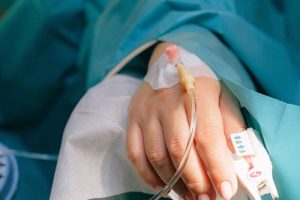
Picture courtesy: Hospimedica |
Intramuscular Lyophilized powder for Injection
| This is the route of administration where lyophilized powder for injection is subjected to the muscle of the body. This is a common route where a longer needle is required as muscle is mostly present deep inside the skin and fatty tissues including the upper arm, hip, or muscle of the buttock. | 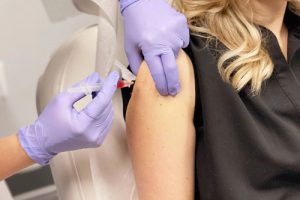
Picture Courtesy: Revive at the group |
Subcutaneous lyophilized powder for injection
| It refers to the administration of lyophilized powder for injection into the beneath dermis in loose connective tissues where small blood vessels take medication and circulate in the body. | 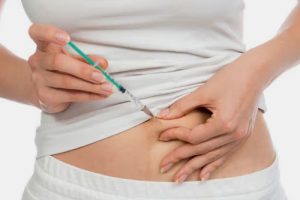
Picture Courtesy: Healthline |
Intrathecal lyophilized powder for injection
| It is the administration of medication into the subarachnoid space surrounding the spinal cord. The needle is subjected to insertion into the lower spine in between vertebrae. | 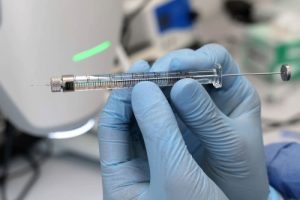
Picture Courtesy: JoVE |
7.What are the process steps for Lyophilized Powder for Injection?
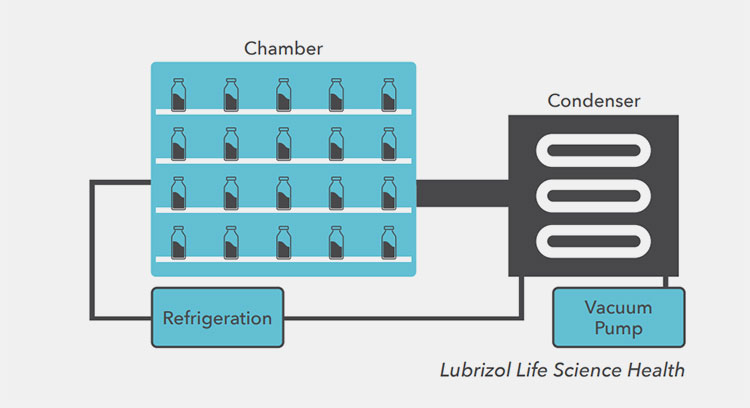
The Lyophilization Process
The preparation of lyophilized powder for injection is based on the principle of sublimation. Do you know what exactly sublimation is? It is the phase where a solvent is transformed to gas without becoming in a phase of liquid. The working of this process is broken down into three main processes and each has critical manipulation related to temperature and pressure.
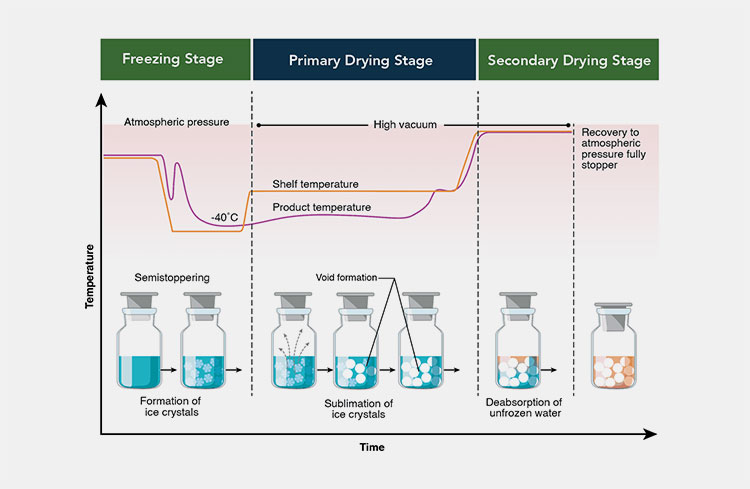
Processing of lyophilized powder for injection-picture courtesy: Pharmaceutic International
STEP 1: Pre-Freezing
In this phase, you will subject the solvent inside the machine (lyophilizer), where it has to be frozen by gently dropping the temperature (-40 degrees) and forming the ice crystals. This is a significant step as by crystalline form can be subjected to a sublimation process. If it is not achieved then evaporation can take place where it fails to attain the preservation properties of the active drug like in sublimation. In the case of the large crystalline structure will allow faster sublimation because greater molecules of water are left out of the sample.
STEP 2: Primary Drying- Sublimation
This is the major phase and here you initiate the freeze dryer with the vacuum pump and lowered pressure. In this phase, the evaporation of cooling items starts and around 93% of water molecules get evaporated or sublimated. The primary drying may take from hours to some days which mainly depends on the sample type and requirement of heat input.
STEP 3: Secondary Drying- Desorption
A secondary drying phase, where the remaining water molecules or moisture content has to be released by providing additional heat that leaves 2% of moisture content and makes the sample capable of preserved for a long period of time. The type of heat is employed known as ‘an isothermal desorption’ which takes place by reduced pressure and temperature is higher than the primary drying phase. The prime aim is to reduce moisture content so that the sample can be shown to have good preservative action.
8.What Equipment is Needed for Lyophilized Powder for Injection?
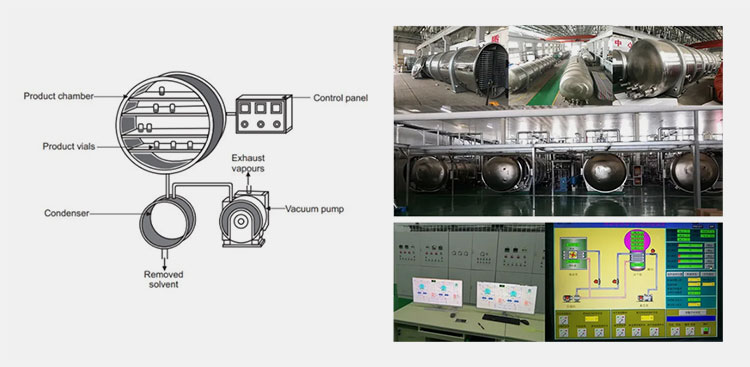
AIPAK ENGINEERING lyophilizer
The main equipment utilized for making lyophilized powder for injection is a ‘lyophilizer’ or ‘freezer dryer’ that functions in a way to evaporate or eliminate the moisture content from the product following the principle of sublimation. This machine is capable of working in reduced pressure and a vacuum with gentle heating to carry out the process.
There are the following parts a condenser, vacuum pump, exhaust evaporator, and shelves inside the chamber. It allows the maximum entrapment of water molecules via stable inside conditions by utilization of parts such as a vacuum pump, exhaust evaporator, and solvent removal.
You can just place the vial with the sample on the shelf of the apparatus put the settings to run the process and receive the sample upon completion of the process notified by the machine.
9.What is the production line of Lyophilized Powder for Injection?
A large assortment of machines is integrated into the production line for lyophilized powders for injection. Different functions from washing to sealing are carried out by these machines. Here is the list of machines present in the production line of lyophilized powder for injections:

AIPAK ENGINEERING Production Line for Lyophilized Powder for Injection
Ultrasonic Bottle Washing Machine
In this machine, ultrasonic waves are produced and are used for removing different types of debris, dust, and other contaminants. Vials are placed in the water tank and with the help of ultrasonic waves a strong cleaning action is produced. Sticky materials present on the surface of the vials are removed when high-energy ultrasonic waves hit them.
Washing Machine
Water is circulated in this machine for wet rinsing of the vials. Water for injection is sprayed to dislodge particles present on the walls and base of the vials. Water spray and a combination of compressed air thoroughly wash the vials.
Sterilization Dryer
This machine is used for fully sterilizing the vials. First, the vials are preheated and then transported to high high-temperature chamber, where at increased temperature, microbial contaminants are removed from the lyophilized powder for injection vials. Afterward, the vials are slowly cooled in this machine.
Filling Machine
It performs the filling of freeze-dried lyophilized powder under aseptic conditions. Different types of barrier technologies are used for gradually and accurately filling the powders into the vials. Different types of peristaltic pumps and time-based filling systems are used for loading solutions in this machine.
Stoppering Machine
Sterile stoppers are placed in the hopper of this machine, from, where they are transferred to the stoppering placing area. This component securely adds the stoppers to the vials.
Capping Machine
After the stoppering machine, the capping machine adds the caps to the stoppered vials. These caps are tightly pressed to seal the caps. This process secures the rubber stopper and forms a strong hermetic seal.
10.What are the packaging products for Lyophilized Powder for Injection?
There are two main types of packaging products for lyophilized powders of injection. These types are stated below for your understanding:
Glass Vials

Glass Vials- Picture Courtesy: Laglorietadel
These are clear or amber-colored vials for lyophilized powder for injection. Glass vials are frequently employed in the pharmaceutical and health care settings for packaging of lyophilized powders for injection because glass offers superior barrier characteristics, has excellent chemical resistance and can easily withstand high temperatures present during sterilization and lyophilization.
Plastic Vials

Plastic Vials Picture Courtesy: PackagingGURUji
They are employed alternatively to the glass vials. Plastic vials are lighter in weight and have breakage resistance. Therefore, it is easier to transport plastic vials. Moreover, plastics are molded into different shapes, creating different design vials. They also provide good chemical resistance. Because of their durability and consistency, it is possible to achieve high-speed filling with plastic vials.
11.How to seal Lyophilized Powder for Injection?

Sealing of Lyophilized Powder for Injection- Picture Courtesy: Tecnosoft srl
Sealing lyophilized powder for injection has paramount importance in preventing contamination of lyophilized powders and preserving their product integrity. Furthermore, sealing is integral in ensuring the sterility and stability of products. There are two main types by which sealing is performed.
| Step 1: Stopping | First, stopping is performed to securely seal the products. It is carried out in the pressure adjustment chamber. In this step, rubber stoppers are added to the opening of vials. These stoppers offer a good barrier that prevents the entry of harmful substances into the lyophilized powders for injection. |
| Step 2 Crimp Sealing | A permanent and safe closure is formed around the stopper in the crimp sealing process. This step is carried out after the stoppering step. Crimping sealing involves the crimping of aluminum caps around the stoppers. |
12.What are the problems during manufacturing Lyophilized Powder for Injection?
Manufacturing lyophilized powder for injection is quite a complicated process in problems are encountered routinely, which results in product instability and loss of yield. Nevertheless, these problems are easily overcome using skilled operators and state-of-the-art equipment.
Some troubleshooting tips for resolving problems associated with manufacturing lyophilized powder for injection are:
Presence of Residual Solvent

Presence of Residual Solvent
| Causes | Solutions |
| Solvents are not fully removed during the drying step.
Inadequate lyophilization settings. No excipient is used to assist the lyophilization process. |
Optimize drying stages to ascertain the complete elimination of solvents.
Test and reset lyophilization parameters. Use excipients to increase the rate of lyophilization. |
Inaccuracy in Fill Volume

Inaccuracy in Fill Volume- Picture Courtesy: NEB
| Causes | Solutions |
| Filling equipment is defective or damaged.
Improper calibration of filling devices. There are variations in the viscosity of the solution. Presence of foaming. |
Maintain and repair filling equipment.
Properly calibrate the filling devices. Ensure uniformity in the viscosity of the solution. Utilize foaming agents if required. |
Stopper Pop-up

Stopper Pop-up- Picture courtesy: Buchi
| Causes | Solutions |
| Incorrect settings of stoppering parameters.
There is insufficient vacuum at the stoppering step. |
Adjust the settings of stoppering parameters to ensure the correct stoppering process.
Fix the vacuum settings so that there is sufficient generation of vacuum during the stoppering step. |
Contamination of Product

Contamination of Product- Picture Courtesy: Bioprocess Online
| Causes | Solutions |
| Presence of contaminants during filling or stoppering process.
Introduction of particles from rubber stopper. |
Maintain sterility during the filling and stoppering processes.
Use pre-sterile rubber stoppers. |
Breakage of Vial

Breakage of Vial- Picture Courtesy: The Tennessee Tribune
| Causes | Solutions |
| High thermal stress during the lyophilization process.
Rough treatment during handling and transportation. |
Use high-quality vials that can tolerate thermal stress.
Employ gentle handling techniques and utilize cushioning materials during transportation. |
Conclusion
With the evolution in pharmaceutical manufacturing, new and sophisticated techniques of drug administration have been introduced, with one being lyophilized powder for injection. Lyophilized powder for injection is the ideal way to preserve the efficiency of active ingredients. Moreover, it is convenient for both physicians and patients. All these advantages are achieved by maintaining sterility during the manufacturing process and this sterility is only possible by using cutting-edge equipment. So, if you want complete sterility and automation in your production plant then consider buying devices related lyophilization process from AIPAK ENGINEERING. We are the experts in establishing injection solutions production lines.
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp:+86 18171018586

Tell us your material or budget, we'll reply you ASAP within 24 hours
Vial lyophilization powder injectable powder production line Related Products