MMR Vaccine Production: The Complete FAQ Guide In 2025
Vaccines are a way to prevent diseases. Have you received vaccines when you were a child? Measles virus, Mumps virus, and Rubella virus can occur all year round, with a high incidence in winter and spring, and are highly contagious. To address these diseases, MMR vaccines have been developed.
Do you know the production process of MMR vaccine? Do you have any side effects with this vaccine? I think you must be very concerned about these issues. Let’s take a look for the complete FAQ guide of MMR vaccine production together.
1.What Is the Definition of MMR Vaccine?

MMR Vaccine - Sourced: MSU Denver - Early Bird
The MMR vaccine, also known as the MMR combined attenuated live vaccine, is a combination vaccine used to prevent three diseases: measles, mumps and rubella. The MMR vaccine was first introduced by Dr. Murray Ha in 1971 Developed by Maurice Hilleman, after years of improvement and refinement, it has become an extremely safe and effective vaccine for preventing diseases.
| Description | Route of transmission | |
| Measles | Measles, is characterized by fever and rash, accompanied by runny nose and tears. More seriously, it can cause pneumonia and cranial infections, as well as lead to blindness in children and miscarriage in pregnant women. | Spread with coughing, sneezing, or any close contact with the patient. |
| Mumps | Mumps can cause fever, headache, and even meningitis, orchitis, ovarian inflammation, etc. | Spread through coughing, sneezing, or contact with the patient’s saliva. |
| Rubella | Rubella, an acute respiratory infectious disease, mainly causes fever and pharyngitis. Adults infected with rubella are prone to rash and chronic arthritis. | Spread through coughing, sneezing, and contact with the patient’s saliva. |
2.What Are the Ingredients of MMR Vaccine?
Ingredients of MMR Vaccine - Sourced: Blue Shield of California
Generating protective effects through vaccination is a complex process. Simply put, it is the process of injecting specially treated viruses into the human body to induce resistance without causing illness. But do you know what ingredients are in the vaccines that are injected into the body?
| Ingredients | Description |
| Attenuated live vaccine | The virus is non pathogenic after treatment, but it can trigger an immune response. |
| Adjuvant | It can help us stimulate the immune system and enhance the immune response to vaccines. |
| Stabilizer | Sugar, gelatin, and other food grade safe substances can protect vaccines during transportation and storage. |
| Preservative | Modern technology no longer requires the addition of thiomersal and has almost no toxicity to the human body. |
3.What Are the Main Steps of MMR Vaccine Production Process?
The MMR vaccine is manufactured to prevent infectious diseases and is a very important preventive measure. To ensure the safety of vaccines, it is necessary to produce them according to a series of precise manufacturing processes.
Step 1: Selecting pathogen
Screening for live viruses of measles, mumps, and rubella, selecting suitable pathogens with good antigenicity, representative characteristics of prevalent strains, and strong growth ability.
Step 2: Bacterial cultivation

Bacterial Cultivation - Sourced: Technology Networks
Firstly, obtaining the leprosy pathogen and cultivating it in cell culture medium to prepare a large number of pathogenic bacteria.
Step 3: Water treatment
The water used for vaccine production needs to be pre treated first, then subjected to secondary permeation, and finally deionized. Only the water obtained through first, second, third, fourth, fifth, and sixth distillation can be truly used for vaccine production.
Step 4: Vaccine mixture
Mixing is the use of vaccine raw materials, which are diluted at a certain ratio and mixed at high speed to achieve complete mixing of ingredients. When mixing, it is necessary to consider the shelf life and antigen strength to ensure safety and effectiveness.
Step 5: Vaccine sterilization

Vaccine Sterilization - Sourced: The Denver Post
Sterilizing the MMR vaccine to eliminate microorganisms and ensure its safety and effectiveness. During the vaccine production process, there may be contamination due to environmental or human factors.
Step 6: Vaccine filling and packaging.
The prepared vaccine will be filled into appropriate containers and packaged to ensure its stability and safety during transportation and storage. Subsequently, the vaccine will be sent for clinical trials or distributed to medical institutions for use.
Step 7: Quality control
In order to ensure that its quality meets international standards, the quality of the prepared MMR vaccine should be strictly controlled. Samples from each final vaccine batch should be evaluated. All tests and specifications must be approved by regulatory authorities. The final vaccine specifications are formulated and proven reasonable by the manufacturer.
4.What Machines Can Be Used in the MMR Vaccine Production?
What are the different types of vaccine production processes, including inactivated vaccines, attenuated live vaccines, recombinant protein vaccines, and viral vector vaccines. MMR vaccine is a live attenuated vaccine. Do you know the process?
| Machines | Description |
| Bioreactor
Bioreactor - Sourced: INOXPA USA |
A bioreactor is a device that simulates the internal environment of an organism and is used for large-scale cultivation of cells or microorganisms, which can be used to cultivate MMR vaccines. |
| Centrifuge
Centrifuge - Sourced: IVF Store |
Centrifuges are necessary equipment for separating different components in a mixture and preparing vaccines. In the vaccine production process, centrifuges are commonly used to separate cells and cell debris, separate viral and non viral components, and remove small molecule impurities. Centrifuges are divided into low-speed centrifuges, high-speed centrifuges, and high-speed centrifuges according to their rotational speed and capacity. |
| Purified water treatment equipment
Purified Water Treatment Equipment |
MMR vaccine production requires purified water. The purified water treatment equipment can automatically control and adjust various parts of the purified water equipment, achieving automated operation of the equipment. Through sedimentation and filtration, impurities and microorganisms in the water can be removed. |
| Vacuum emulsifying mixer
Vacuum Emulsifying Mixer |
Vacuum emulsifying mixer is a device used to break down the cell wall and release intracellular substances. This is very important for the development and production of MMR vaccines, as homogenization technology can be used for the homogenization of vaccine raw materials to ensure inter batch consistency. It is carried out in a vacuum environment, avoiding contact between the material and oxygen in the air. |
| Pharmaceutical autoclave
Pharmaceutical Autoclave |
Large pharmaceutical autoclave are typically used for mass production, with large capacity and efficient sterilization capabilities. This type of sterilization cabinet generally uses steam or dry heat sterilization methods, which can quickly and effectively kill bacteria and microorganisms. Its size is relatively large and usually needs to be customized according to the actual production space of the pharmaceutical factory. |
| MMR vaccine filling line
MMR Vaccine Filling Line |
The MMR vaccine is filled in vials and mainly consists of a series of automated equipment, including bottle feeding machines, filling machines, capping machines, labeling machines, etc., which can achieve the entire process of automatic cleaning, filling, sealing, labeling, and output of vials. The MMR vaccine filling line adopts a PLC automatic control system and is made of high-quality SUS304 and 316 stainless steel to meet the strict material requirements of the pharmaceutical industry. |
5.What Is the Effectiveness of MMR Vaccine?
Effectiveness of MMR Vaccine - Sourced: CDC
The duration of vaccine efficacy varies depending on the type and individual differences, but generally speaking, the protective effect of most vaccines can last for many years or even a lifetime.
The MMR vaccine is mainly used to prevent three diseases: measles, rubella, and mumps. After vaccination, it can stimulate the body to produce immunity against measles virus and rubella virus, and is mainly used to prevent these three diseases.
The immune effect of the MMR vaccine can generally last for more than 10 years. The MMR vaccine has an efficacy rate of 97% for measles, 88% for mumps, and 97% for rubella.
After receiving this vaccine, it generally does not have any adverse effects on the body, but some patients may experience adverse reactions such as fever, rash, cough, diarrhea, etc., which are usually transient and can be relieved on their own.
6.What Is the Duration of Protection of MMR Vaccine?
Vaccines play a preventive role, not lifelong immunity. Do you know the duration of protection of MMR vaccines?
Duration of Protection of MMR Vaccine - Sourced: Public Health Command-Pacific
After full vaccination, the protection rate against measles and rubella is over 95%, and the immunization time is maintained for at least 10 years. The protection rate against mumps can reach about 70% with one dose and about 85% with two doses.
After receiving this vaccine, it generally does not have any adverse effects on the body, but some patients may experience adverse reactions such as fever, rash, cough, diarrhea, etc., which are usually transient and can be relieved on their own.
7.What Are the Side Effects of MMR Vaccine?
After receiving the MMR vaccine, there may be some adverse reactions that require attention.
Local reaction
The vaccination site may experience redness, swelling, pain, and hardening. It usually appears within hours to days after vaccination, and is generally mild with a short duration.
Fever

Fever - Sourced: UC Davis Health
Some babies may experience fever after receiving the MMR vaccine, usually low-grade or moderate fever. It often appears within 7-10 days after vaccination and may last for 1-2 days.
Rash
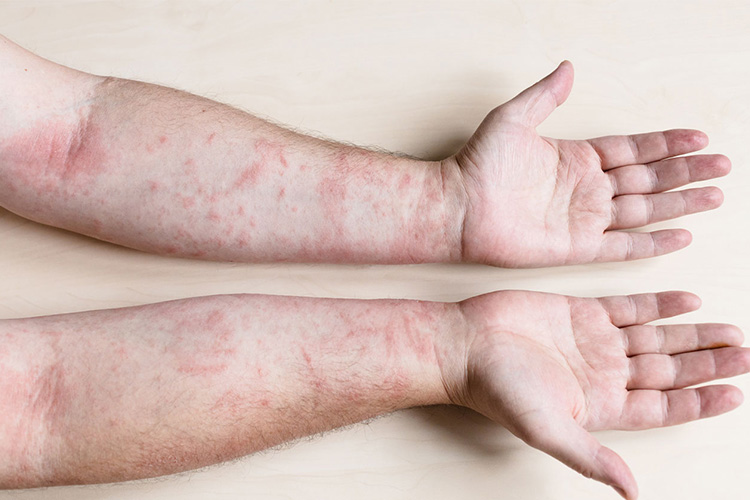
Rash - Sourced: Dermatology Associates
A small number of babies may develop a rash, manifested as scattered red papules. It usually appears within a few days to two weeks after vaccination. After receiving the MMR vaccine, observation should be conducted at the vaccination site.
Enlargement of parotid and salivary glands
Mild swelling of the parotid and salivary glands may occur after vaccination, and usually improves on its own within one week. If necessary, seek medical treatment at a hospital.
8.What Are the Current Recommendations For the Use of MMR Vaccine?
The MMR vaccine can prevent three infectious diseases, and attention should be paid when administering the MMR vaccine.
Before vaccination
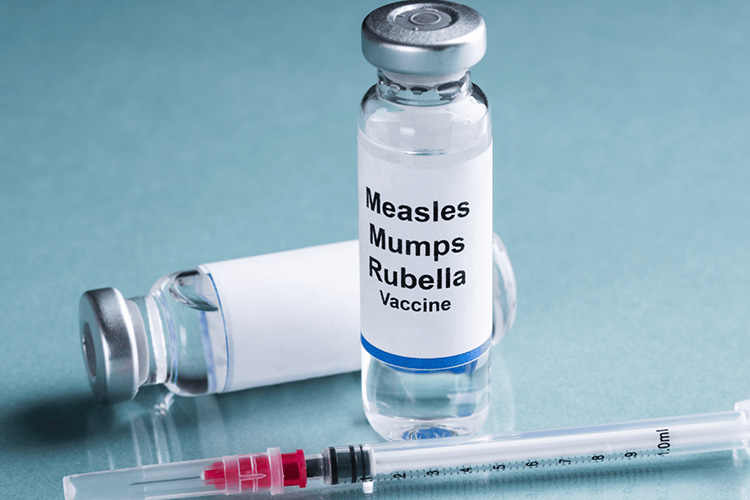
MMR Vaccine Vial - Sourced: Immunology Explained
MMR vaccine vials with cracks, unclear labels, or expired or expired products are not allowed to be used. After adding sterilized injection water to the vaccine, gently shaking the medicine can immediately dissolve it. If the MMR vaccine cannot be used immediately, it should be placed in an environment of 2-8 ℃ and used within 1 hour. The remaining vaccine needs to be discarded. another
Do not allow disinfectant to come into contact with the vaccine during the opening and injection of the external MMR combined attenuated live vaccine.
After vaccination

After Vaccination - Sourced: Midwest Express Clinic
When the vaccinated person has just received the vaccine, they need to be observed for 30 minutes. Usually, there will be slight pain and swelling in the local area, which is a normal phenomenon and does not require special treatment. After about 3-5 days, the discomfort will ease on its own. After receiving the MMR combined with attenuated live vaccine, it is also not advisable to take a shower immediately to avoid increasing the risk of skin infection.
After receiving the MMR combined with attenuated live vaccine, the vaccinated person may experience a transient fever reaction, which usually takes 1-2 days to recover and does not require treatment. They may also experience rash, salivary gland enlargement, and other conditions. Allergic rash, anaphylactic shock, joint pain, swelling and other symptoms may also appear after vaccination, but they are relatively rare.
9.What Are the Administration Method of MMR Vaccine?
The MMR vaccine is generally administered through subcutaneous injection and intramuscular injection.
Subcutaneous injection
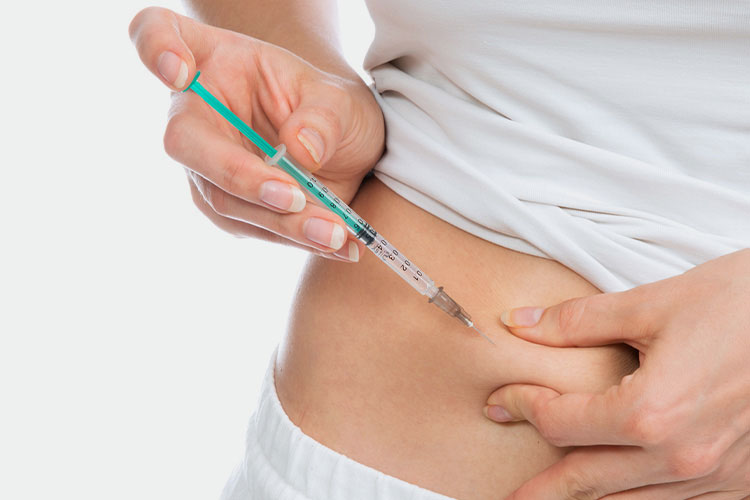
Subcutaneous Injection - Sourced: Cro
It is usually performed in areas with abundant subcutaneous fat such as the abdomen, front or outer thighs. This injection method is relatively mild and suitable for people who have certain concerns about intramuscular injection, which can reduce discomfort and pain at the injection site.
Intramuscular injection
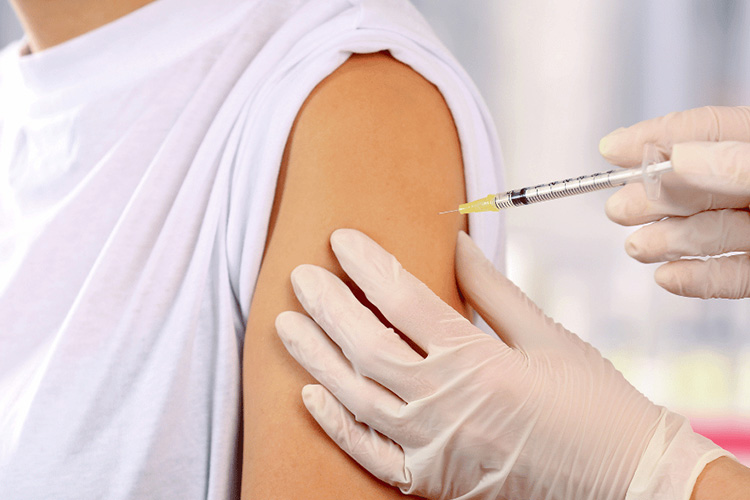
Intramuscular Injection - Sourced: Drip IV Therapy
Usually, the deltoid muscle in the upper arm is chosen for injection, as this area has abundant muscles that are conducive to the absorption and distribution of vaccines, ensuring that the vaccine can effectively stimulate the body to produce an immune response, thereby achieving the goal of preventing measles, mumps, and rubella.
10.What Are the Contraindications and Precautions for MMR Vaccine?
When receiving the MMR vaccine, it is important to pay attention to some contraindications to avoid vaccination failure or adverse reactions.
Light diet
The MMR vaccine is mainly used to prevent measles, mumps, and rubella. During and before vaccination, it is important to maintain a light diet and avoid spicy and stimulating foods.
Allergy prohibition
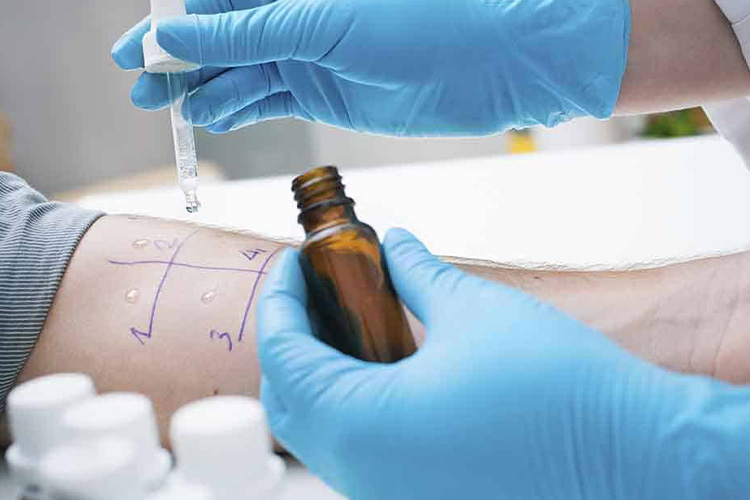
Allergy Prohibition - Sourced: Allergy & Asthma Network
If a patient has recently experienced an allergic reaction, vaccination is usually not suitable as it may affect the efficacy of the medication and worsen the allergic condition.
Avoid getting wet
The place where the MMR vaccine is injected may become infected if it gets wet. After vaccination, you should avoid getting the wound wet to prevent infection and affect recovery.
Vaccination taboo population
The MMR vaccine is a live vaccine and pregnant women should not receive it. If a woman plans to conceive, she should wait at least one month (preferably three months) after receiving the vaccine before attempting to conceive.
Adults with weaker immune systems, such as those undergoing immunosuppressive therapy, should also receive vaccination under the guidance of a doctor.
11.What Are the Recommended Catch-up Vaccination Schedule for MMR Vaccine?
MMR is a three in one vaccine for measles, mumps, and rubella, which can prevent all three diseases simultaneously. These three diseases have high infectivity, especially measles, which may cause serious complications; Rubella is extremely harmful to pregnant women and fetuses.
MMR vaccine usually requires two doses, with reference to the vaccination time:
| First dose: | It is recommended to get vaccinated at 12-15 months of age. |
| Second dose: | It is recommended to get vaccinated between the ages of 4-6. |
12.What Quality Regulations Are Required for MMR Vaccine Production?
The production of MMR vaccine involves multiple biological processes, which face the risks of contamination and introduction of impurities during the production process. Whether in terms of scale or among multiple manufacturers, the necessary quality can only be achieved through standards.
FDA

FDA - Sourced: KFF Health News
The manufacturing process and quality control system of MMR vaccines also need to comply with FDA requirements. The applicant needs to provide detailed information about production processes, raw materials, quality testing, and other aspects. The submitted application should also include the drug label and instructions for use of the vaccine, ensuring that medical professionals and patients understand the correct usage methods and precautions.
World Health Organization (WHO) guidelines
WHO and other international organizations have developed a series of vaccine quality standards, such as the Guidelines for Vaccine Production and Quality Control. These standards provide a basic basis for vaccine regulation in various countries, and the production of MMR vaccines must be strictly controlled.
GMP

GMP - Sourced: A. Ebbecke Verfahrenstechnik
The GMP requirements for MMR vaccine production run through all stages of the entire process. The production workshop needs to maintain a specific level of cleanliness and environment. Personnel entering the production area must strictly follow the changing procedures.
Production equipment should be regularly maintained and calibrated. Equipment selection should meet the requirements of vaccine production processes. Raw material procurement should come from qualified suppliers. Strictly follow the acceptance standards when receiving raw materials. Different batches of raw materials should be stored and managed separately.
13.How to Ensure the Safety And Efficacy of the MMR Vaccine?
Before the MMR vaccine enters human clinical trials, extensive preclinical research is required. What needs to be done to ensure the safety and efficacy of the MMR vaccine?
Strict production standards

Strict Production Standards - Sourced: GMP.COM
MMR vaccine manufacturers need to follow strict Good Manufacturing Practices (GMP) for drug production. This includes environmental control of the production plant, such as maintaining a clean production workshop, controlling temperature, humidity, and air quality to prevent microbial contamination. Production equipment also needs regular maintenance and calibration to ensure the accuracy and stability of the production process.
Quality control of raw materials
Strict screening and quality testing of raw materials for MMR vaccine production. For example, for viral vaccines, the cell matrix used to cultivate the virus must come from qualified cell lines and the cells must be tested for contamination (such as viruses, bacteria, mycoplasma, etc.). The purity of raw materials is also crucial, as any impurities may affect the quality and safety of vaccines.
Finished product testing

Finished Product Testing - Sourced: NJ Labs
Each batch of MMR vaccine must undergo comprehensive quality testing after production is completed. This includes testing the efficacy of vaccines, that is, whether their ability to trigger immune responses meets standards; Testing the safety indicators of vaccines, such as detecting the presence of residual harmful substances (such as chemical reagents used in the production process) and microbial contamination.
ADR monitoring
Establishing a MMR vaccine adverse reaction monitoring system, such as the Vaccine Adverse Event Reporting System (VAERS) in the United States and the Drug Adverse Reaction Monitoring System in China. Medical staff, patients, and their families can report any adverse reactions that occur after vaccination through these systems. The monitoring department will analyze these reports to determine whether the adverse reactions are related to the vaccine, as well as the incidence and severity of adverse reactions.
14.What Are the Storage Requirements For the MMR Vaccine?
The storage and preservation of MMR vaccines is very important, as it directly affects the effectiveness and safety of the vaccines. Here are the precautions for storing and preserving vaccines.
Temperature control

Temperature Control - Sourced: First American Home Warranty
Different types of vaccines need to be stored at different temperatures. Generally speaking, MMR vaccines should be stored refrigerated (2-8 ℃ or 35-46 ℉). It is necessary to ensure that the temperature is controlled within the required range to avoid excessive or insufficient temperature affecting the quality of vaccines.
Condition monitoring
MMR vaccines require regular monitoring of environmental conditions, including temperature and humidity, during storage. Temperature and humidity meters and other instruments can be used for monitoring, and the monitoring results can be recorded. If the temperature or humidity exceeds the specified range, timely measures should be taken to adjust it to avoid vaccine damage.
Packaging protection
MMR vaccines need to be kept in their original packaging during storage to protect the active ingredients from external influences. The original packaging is usually carefully designed to provide a certain degree of insulation and moisture resistance. Do not open or cut the original packaging of the vaccine to avoid affecting its quality.
Staying out of the sun

Staying Out of the Sun - Sourced: Yahoo
MMR Vaccines need to be stored in a dry, cool, and shaded place, away from direct sunlight. Ultraviolet rays and high temperatures in sunlight can have adverse effects on vaccines, potentially leading to degradation of vaccine components, loss of active ingredients, or bacterial contamination.
Isolated storage
Different types of vaccines should be stored in isolation to avoid contact and contamination. This can prevent cross reactions and contamination between vaccines, ensuring the purity and effectiveness of the vaccines.
Conclusion
MMR vaccine is one of the vaccines administered to children, which can reduce the infection of diseases and enhance immunity. After reading this article, what is your understanding of the efficacy, adverse reactions, and production process of MMR vaccine? If you want to learn more about MMR vaccine, you can contact AIPAK Engineering at any time
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours







