Lyophilized Vial:The Complete FAQ Guide In 2025
Lyophilized powder is widely used in biological products, vaccines, and other high-value drugs. However, the packaging of lyophilized powder is crucial for ensuring drug quality and extending shelf life. Lyophilized vial, as an important carrier of lyophilized technology, play a crucial role in modern industrial production. Through scientific lyophilized technology, vial can effectively maintain the activity, stability, and quality of products, thereby meeting the needs of various fields.
How much do you know about lyophilized vial? Do you know the production process of lyophilized vial? Next, let’s take a look at the complete FAQ guide on lyophilized vial.
1.What Is the Definition of Lyophilized Vial?

Lyophilized Vial - Sourced: Pharmaceutics International
Simply put, lyophilized vials are vials used to fill lyophilized(freeze-dried) products. These lyophilized products include vaccines, serum, etc. Lyophilized technology can remove moisture from these products, retain their activity, and extend their shelf life. This kind of vials come in brown and transparent types.
2.What Are the Benefits of Lyophilized Vial ?
Why are there many lyophilized vial in the pharmaceutical industry? Actually, it has many benefits.
Diverse specifications
There are various types of lyophilized vials, ranging from small bottles with a capacity of 2ML to large vials with a capacity of over ten liters, from round, square, to irregularly shaped and handled vials, as well as opaque opaque opaque glass bottles, and so on.
Avoiding light

Avoid Light- Sourced: Berkshire Sterile Manufacturing
Vials can have a light shielding effect, effectively resisting sunlight exposure. In summer, the sunlight is more abundant, which accelerates the oxidation of drugs. Some chemically unstable drugs are prone to decomposition when exposed to light, which not only reduces their activity but also increases their toxicity, seriously affecting their effectiveness.
High transparency and ease of inspection
High transparency facilitates drug inspection by allowing for clear visibility of the lyophilized powder inside bottles during production and quality control. This enables prompt detection of any potential abnormalities, thereby enhancing both production efficiency and drug safety, guaranteeing that each bottle adheres to quality standards.
Good barrier performance

Good Barrier Performance - Sourced: GEA
Glass materials have good barrier properties, which can effectively prevent oxygen and other gases from invading lyophilized products, while also preventing volatile components of the contents from evaporating into the atmosphere.
3.What Is the Production Process of Lyophilized Vial?
The production of lyophilized vial requires many processes, and each step is coordinated with each other. The following text provides a detailed introduction to each step.
Cleaning and preparing
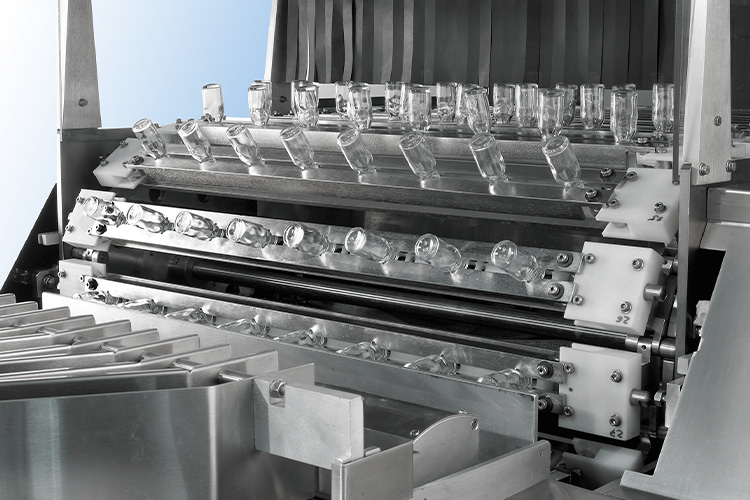
Cleaning the Vials - Sourced: Cozzoil Machine Company
Before production, tools such as rubber stoppers and vials need to be cleaned and sterilized in D-level or C-level areas. Usually, vialss are sterilized by dry heat and the rubber plug is sterilized by wet heat.
And then, operators should prepare the raw materials for lyophilized vial, which mainly include active ingredients, moisturizers, stabilizers, etc. In the stage of preparing raw materials, it is necessary to strictly screen high-quality raw materials and conduct quality testing to ensure that the raw materials meet relevant standards and requirements.
Sterilization and filtration

Vial Sterilization - Sourced: Groninger
The filtering of medicinal liquids usually adopts a two-stage filtration system to achieve A/B levels. The filter uses SIP sterilization and requires integrity testing of the sterilization filter element before and after use.
In addition, it is necessary to control the time interval between the preparation and sterilization and filtration of the medication, which is based on considerations of microbial control and medication stability. The sterile retention time is determined by simulating the filling time of the culture medium, and the stability of the drug solution is confirmed through product process validation.
Filling and stoppering

Filling and Stoppering - Sourced: Berkshire Sterile Manufacturing
The sterile filling area is located in the A-level zone and is a key high-risk area for the production of non final sterilized products. To reduce risks, it is necessary to reduce personnel intervention and control drug exposure time.
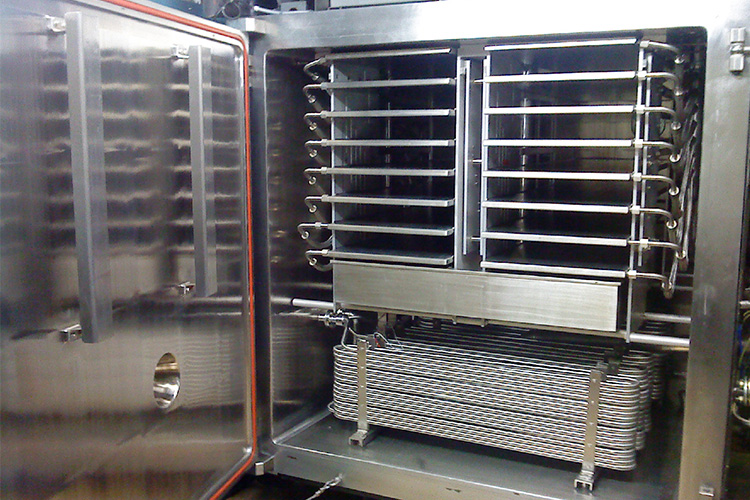
Freeze drying(lyophilization)

Freeze Drying - Sourced: ThoughtCo
Freeze drying is the core process of lyophilized powder production. At this stage, advanced lyophilized equipment and technology are used to freeze dry the filled vials. During the freeze-drying process, it is necessary to strictly control key parameters such as temperature and humidity to ensure that the product is dried under optimal conditions. After freeze-drying treatment, the moisture in the raw materials is removed, and the product can be stably stored.
Capping
After the lyophilized vial enters the capping area, the capping head descends and presses the vial cap tightly. Under the rotating action of the capping head, the cap tightly adheres to the vial mouth, forming a seal. After the capping is completed, the capping head rises and the vial continues to move along the conveyor belt, completing the entire capping process.
Labeling
Finally, labeling the vial based on the product information, such as production date, product information, etc. During the labeling process, the operators should avoid moisture, and pollution.
4.What Machines Are Used in the Production of Lyophilized Vial?
The production of lyophilized vial is similar to a general vial production line, but there is an additional freeze-drying process.
Ultrasonic vial washing machine

AIPAK Ultrasonic Vial Washing Machine
Equipped with a high degree of automation, the ultrasonic vial washing machine is used for cleaning lyophilized vials, among 2-100ml vials. This machine uses ultrasonic technology to generate strong cleaning force through high-frequency vibration, which can thoroughly clean the dirt and impurities inside and outside the vial, guaranteeing that the vial’s cleanliness aligns with production standards..
Sterilization tunnel

Sterilization Tunnel
This sterilization tunnel mainly uses the principle of high temperature dry heat to kill microorganisms. During the sterilization process, the lyophilized vials that need to be sterilized are placed in a sealed sterilization tunnel, and then the temperature is adjusted to a high temperature state, usually 160℃, and maintained for 2 to 4 hours.
During this period, high temperature will kill all bacterial inside the sterilization tunnel, achieving the purpose of sterilization.
Vial filling and stoppering machine

AIPAK Vial Filling And Stoppering Machine
By adopting advanced measurement technology and full servo drive, the filling and stoppering machine can guarantee accurate and error free material quantity for each filling, thus meeting the strict precision requirements of pharmaceuticals and chemical products.
The filling head descends and inserts into the vial mouth, starting to fill the material. The filling volume can be precisely controlled through the measuring system on the equipment. After the filling is completed, the capping mechanism covers the cap on the vial mouth and ensures the tightness of the cap by rotating or pressing.
Freeze drying machine (Lyophilizer)
Freeze Drying Machine - Sourced: Exapro
The working principle of a freeze drying machine goes through four stages: pre freezing, sublimation, condensation, and collection. The moisture in the material is frozen into ice crystals in a low-temperature environment, and then the ice crystals are directly sublimated into water vapor by heating in a vacuum state.
Finally, the water vapor is condensed into water and discharged through a condenser to achieve the purpose of drying the material. This technology can maximize the preservation of the nutritional content, taste, and original characteristics of materials.
Capping machine

AIPAK Capping Machine
Adopting precision design, the capping machine can adapt to different specifications of lyophilized vial caps and ensure sealing quality. Equipped with sensors, it is capable of real-time monitoring of parameters during the capping procedures.
Besides, the machine has automatic alarm and shutdown functions. Once any abnormal situation is detected, it will be dealt with immediately to ensure production safety.
Labeling machine

AIPAK Labeling Machine
The vial labeling machine has become an ideal choice for the pharmaceutical packaging industry due to its excellent performance and precise process. It adopts advanced automation technology to ensure precise alignment and perfect fitting of labels. By using an HP inkjet printer, the labeling process can be synchronized with the identification of the third phase code.
In addition, a suction cup type automatic removal system has been selected, equipped with a removal receiving tray, which can save manual sorting time and improve production efficiency.
5.What Are the Common Size of Lyophilized Vial?
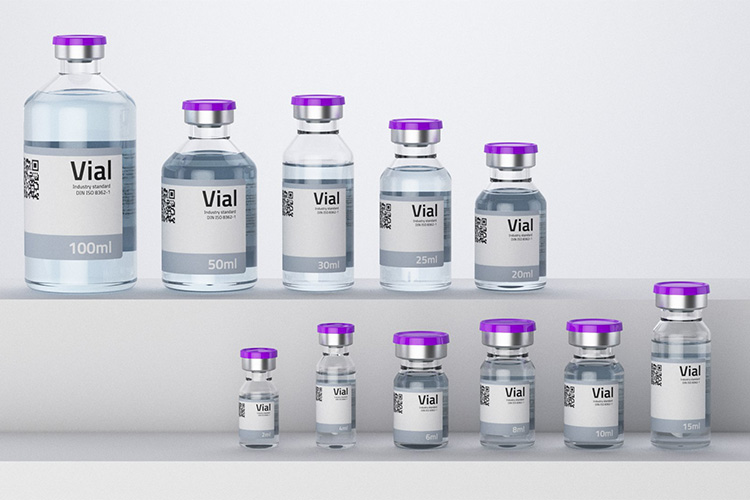
Common Size of Lyophilized Vial - Sourced: TurboSquid
Due to its low cost, vials are the most commonly used in the pharmaceutical industry, and their size varies depending on the manufacturer and product usage, but most vial sizes are relatively fixed.
Usually, the diameter of lyophilized vials is about 35mm, the height is about 60-70mm, and the volume ranges from 2-100 ml.
6.What Are the Main Types of Caps for Lyophilized Vial ?
The cap is related to the sealing of lyophilized vials. What are the common lyophilized vial caps? Next, let me introduce you to three common types.
Screw
Screw is a common cap for lyophilized vials, and the screw of vials has good sealing performance. When the screw cap is rotated, pressure can be applied to fix the diaphragm on the bottle cap.
Crimp

Crimp - Sourced: Shimadzu
The crimp is usually composed of an aluminum cover and PTFE/silicone diaphragm, which has good sealing and corrosion resistance.
Snap

Snap - Sourced: VWR
The snap product is not suitable for long-term storage and can be used together with the spacer. The snap can also provide a certain sealing effect, and this type of lid can be removed by hand. The top of the snap has an opening for easy use of the automatic sampler.
7.What Are the Common Septum Materials for Lyophilized Vial?
In the field of pharmaceutical packaging, septum usually refers to a thin film material that can block external environmental pollution of drugs.

Septum for Lyophilized Vial
Siliconization
A common coating on the inner wall of lyophilized vials is siliconization, usually treated with materials such as dimethyl silicone oil for silicification.
PTFE
Polytetrafluoroethylene(PTFE) septum has the advantages of the most significant anti voltage strength and breakdown voltage. PTFE septum are used for short-term storage and single injection use.
8.What Methods can be Used to Check the Sealing of Lyophilized Vial ?
The sealing of lyophilized vial is crucial, as it affects the quality and shelf life of the product. If the sealing is poor, the shelf life of the product will be significantly shortened. Therefore, this article introduces several methods for detecting lyophilized vial.
Pressure attenuation
Using the pressure inside the filled vial, this method perform sealing testing. You can place the vial in a vacuum chamber for vacuum extraction, then place it in a sealed chamber filled with gas and observe the pressure changes inside and outside. If the pressure changes, it indicates poor sealing. This method is easy to operate, but it is not suitable for penicillin bottles with high sealing requirements.
Laser particle size detection
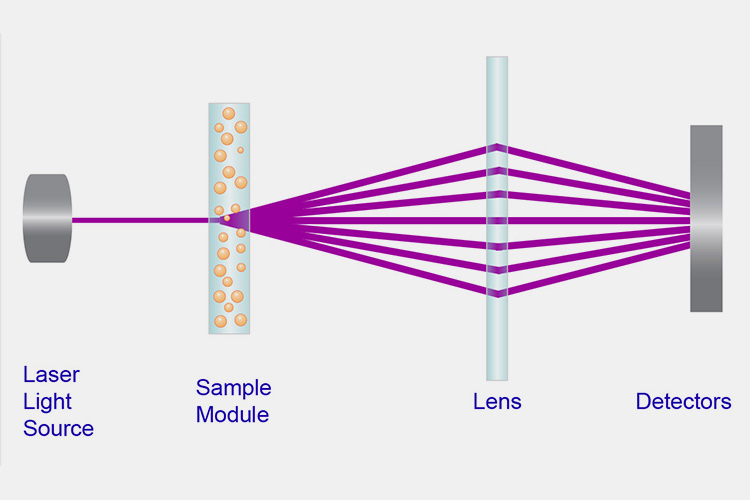
Laser Particle Size Detection - Sourced: Particle Technology Labs
This method detects the sealing of vials by utilizing the principle of laser scattering. The sealing performance is mainly judged by detecting the scattering intensity of the laser passing through the bottle mouth. This method can detect some small vulnerabilities or cracks with high detection accuracy, but the operation is complex and requires professional equipment and personnel.
9.What Are the Common Issues and Solutions of Lyophilized Vial Production?
In industrial production, lyophilized vials may also encounter some problems. Next, let’s take a look at common issues in production.
Broken lyophilizedvials

Broken Lyophilized Vials - Sourced: Journal of Pharmaceutical Sciences
If there are many broken lyophilized vials, it is likely a quality issue with the vials, such as some being thin and prone to breakage. You can replace vials of the same specifications from different manufacturers for production.
You can check if the capacity of the penicillin bottle is too high. If it is too high, you can replace it with a larger capacity penicillin bottle.
Melting
This situation occurs when the initial drying stage is over, but there is still ice at the bottom of some vials. During the secondary drying process, the remaining ice cannot be removed. When this process is completed, the ice that has not been properly removed will melt and wet the dry product, forming some voids. Therefore, a longer initial drying time needs to be set. And you should increase the safe time between initial and secondary drying.
Shrinkage and bubbling

Shrinkage and Bubbling - Sourced: Journal of Pharmaceutical Sciences
Due to the rapid heating during the sublimation stage, excessive temperature can cause local melting, resulting in the evaporation of liquid into gas, causing a reduction in volume, or the dissolution of dried products into the liquid, causing a reduction in volume. Severe melting can lead to bubbling.
Therefore, if such a problem arises, you need to lower the temperature of the sublimation stage, extend the time of the sublimation stage, and increase the vacuum degree of the freeze-drying box, controlling the product temperature to be at least 5℃ lower than the eutectic point or disintegration point temperature.
Climbing the wall of vial
When the initial drying begins, the unfrozen product locally boils, bursts, and sticks to the inner side wall of the vial from the top, and the temperature of the side wall is lower than that of the product. Therefore, you should add annealing steps for heat treatment. And you can use components to prevent product migration to the top level.
Conclusion
Lyophilized vials play an important role in the pharmaceutical industry, and the production process of lyophilized vials involves many machines. After reading this article, do you have a deeper understanding? If you have any further questions about lyophilized vials, you can contact AIPAK Engineering at any time
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours

