Hormone Manufacturing Facility Guidelines: The Complete FAQ Guide In 2025
Are you a manufacturer or an industrial pharmacist? Are you searching for an authoritative content about hormone manufacturing facility? You don’t need to go anywhere, as this guide is crafted in a best way to answer all your queries referred to hormone manufacturing facility guidelines.
Have you felt the importance of hormone manufacturing? Don’t you think this must be carried out in genuine way? This topic tells you about benefits, main components, manufacturing steps, machines, important regulatory bodies and maintenance tips according to hormone manufacturing facility guidelines. Let’s read and learn!
1.What do you mean by hormone manufacturing facility guidelines?
Hormone Manufacturing Facility Guidelines
Are you known to the fact that hormones are fundamental need of your body? When you lack a hormone, you have to supplement it for your better wellbeing. Hormones are delicate substances and their production demands for devoted manufacturing facility. Such production can only be possible when you follow hormone manufacturing facility guidelines.
Do you know what can be hormone manufacturing facility guidelines? These are some rules and regulations that are made to follow them during the hormone manufacturing. Implementation of hormone manufacturing facility guidelines is crucial because you cannot afford any trouble during hormone manufacturing as it turns into a disaster. You always find that these guidelines ensure the safety and sterility of material, equipment and production area of hormone.
2.What can be the benefits of hormone manufacturing facility guidelines?
Have you realized the importance of hormone manufacturing facility guidelines? These guidelines contribute in favor of patients, healthcare system and pharmaceutical industries too. This topic makes you aware about the significant role of hormone manufacturing facility guidelines.
Providing a high quality product:
Providing A High Quality Product-Picture Courtesy: Webmd
Do you have an idea how hormone manufacturing facility helps you to produce a high quality product? When you continue to use high grade material, standardized process, and regulatory compliant equipment, you surely get a high quality product.
After implementing these guidelines, you concentrate on each and every step of manufacturing process that it must be according to mandatory standards. All products are manufactured in controlled environment that results in safe and potent products.
Endorsing the safety of patient:
Endorsing The Safety Of Patient-Picture Courtesy: UW Medicine
Do you know these guidelines allow you to perform hormone manufacturing in a separate place only? Containers, machines and area are kept sterilized and material is procured from documented sources and then do you expect any chance of contamination? Whole framework is designed in such a way to ensure a sterile and potent hormone supplement, which in return endorses the safety of patient.
Keeping staff and environment safe:

Keeping Staff And Environment Safe-Picture Courtesy: Biocon
Do you have any realization that how these guidelines play their part to keep environment and staff safe? You always observe that staff is made obliged to wear a costume in hormone manufacturing facility to keep them safe from any hazardous reactions of hormone and to protect the product from human contamination. They are made well-trained to operate equipment and to manage other requirements of controlled environment.
Hormone manufacturing facility guidelines also ensure environment safety by promoting cautious disposal of waste produced during manufacturing. It is preferred to use environmentally friendly material for secondary packaging of products.
Increasing productivity:
Increasing productivity-picture courtesy: The guardian
Have you thought how hormone manufacturing facility guidelines contribute in increasing productivity? When you follow these guidelines, you make sure to perform every process according to good manufacturing practices. You look for machines that are competent to mandatory standards. You record and document the information of every batch to promote traceability.
When you take above measures, you experience many privileges like you gain cost effectiveness and accountability about hormone manufacturing facility, which leads to increase in productivity.
Boosting economy:

Boosting Economy-Picture Courtesy: InLegal PRO
When you produce a product according to hormone manufacturing facility guidelines, it is confirmed that the final product meets quality compliance and it must be recognized worldwide. You can conveniently export your products due to high quality to different countries and gain profit from that exportation. This brings favor to economy of your country.
Bringing innovation in products:
Bringing Innovation In Products-Picture Courtesy: Kierunik Farmacja
Hormone manufacturing facility guidelines work by strictly implementing the standardized practices in manufacturing facility. You find it very often that the processes are validated to ensure the proper working. These corrections lead to positive growth in such a way that manufacturers seek for innovative ideas to make their production better and satisfying.
3.Do you know the main components of hormone manufacturing facility guidelines?
When you talk about main components of hormone manufacturing facility guidelines, you focus on different aspects that need to be kept maintained according to these guidelines.
| Main components | Information |
| Regulatory compliance:
|
The very first thing you need to notice is that either the guidelines you proposed are meeting the good manufacturing practices (GMP) guidelines or not?
You should look after that the given guidelines are covering every important aspect and they must be competent to regulatory standards suggested by different regulatory bodies like FDA. |
| Manufacturing area:
Picture Courtesy: C&EN |
There are some important things that are practiced like:
You must have a separated are for hormone manufacturing and this area also must be divided in segregated portions for carrying out the process such as a place to handle ingredients, a place to perform mixing etc. If you are carrying out production of different hormones, so make sure they also have separate manufacturing facility. Keep one thing in your mind that this manufacturing area must have clean rooms, where you can maintain aseptic conditions. |
| HVAC system:
Picture Courtesy: NGS Cleanrooms |
Do you sense the importance of HVAC system in hormone manufacturing?
This systems works behind the controlled environment of clean rooms. It provides you fresh air in that manufacturing area and HEPA filters are there to keep air sterile. You may notice that temperature, humidity, presence of microorganism and unwanted particles, all the things are managed by this system. |
| Material & working staff:
Picture Courtesy: Lindstorm Group |
These guidelines also suggested some rules for the material you are using in manufacturing and the staff working in that premises.
Material which is using in manufacturing must be of high-grade quality and sourced from a reliable supplier. Material must be passed in single-directional form to nullify the risk of contamination. You can see that the working staff is restricted to wear gowns, to prevent any hazard to them as hormones can be dangerous. This costume also play role in minimizing the contamination risk. Working staff is trained sensibly about handling the material and machines. Working staff is got educated about the behavior they need to adopt in clean rooms. Waste, which is produced after the completion of batch, is managed expertly by disposing it in a proper way. |
| Machine qualification and validation:
Picture Courtesy: Armein Pharmaceutical Pvt. Ltd. |
Machines are the core of any production, do you agree this?
Machines are inspected for their competence with regulatory standards. You can observe that different means like clean-in-place or sterile-in-place (CIP/SIP) and restricted access barrier system (RABS) are used to keep manufacturing process sterile. Machines are checked for faults according to prepared schedule. Cleaning of machines and production area is carried out by authentic ways, even oxidizers or detergents are used for cleaning. Swab test can be performed to check presence of particles. |
| Manufacturing process:
Picture Courtesy: Packaging Strategies |
The whole process that manufacturing of hormone follows need to be validated, do you know why? As these validations tell you that the process is carried out properly.
Information of every batch must be recorded and documented to avoid trouble in future and also you can trace the problem if it will happen. |
| Quality control:
Picture Courtesy: Zivon Lifecare |
Quality control secures chief place when it comes to authenticity of final product, do you know how?
You perform different tests that confirm about the quality of final product, these tests can be performed to check purity, potency, sterility, microbial content or impurity etc. |
4.What products can be prepared under hormone manufacturing facility guidelines?
Do you know the products that are used to supplement hormones? Hormone manufacturing facility guidelines facilitates the preparation of many useful products, which have significant role in treating a disease condition or promoting the good health condition. Few examples are stated below:
| Products | Information |
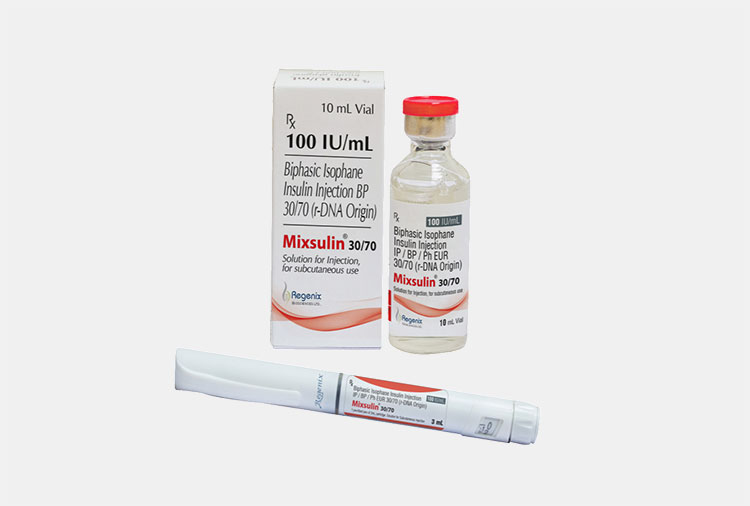
| Insulin
|
Have you heard about insulin? It is commonly used hormone, taken to manage diabetes Mellitus.
It is a peptide hormone and available in market with different names like regular insulin, insulin glargine etc. It is administered by subcutaneous (SC) route but in emergency you can give it by intravenous (IV) route. |
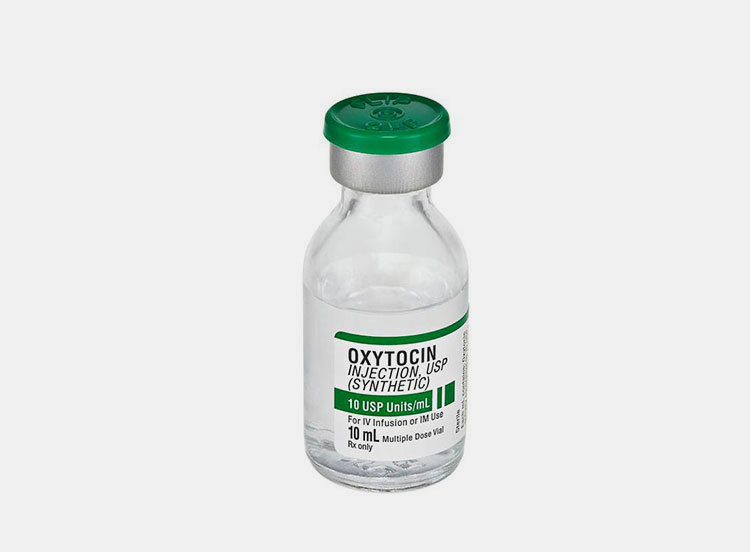
| Oxytocin
|
Oxytocin is known to be used for initiating or increasing uterine contractions and this hormone has enormous value in gynecology.
It has ability to fasten the vaginal delivery and minimizing the bleeding after child birth. You can introduce it in body by intra venous (IV) or intra muscular (IM) route. |
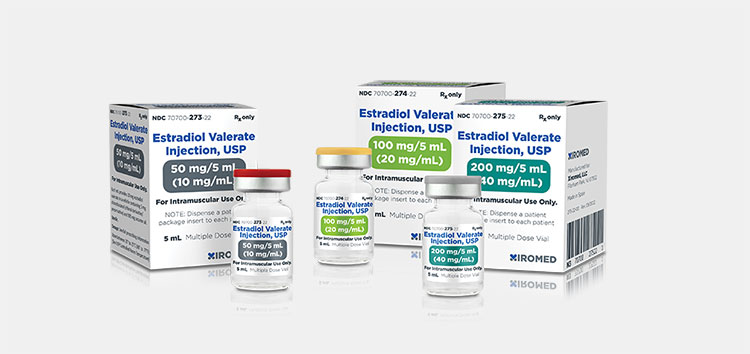
| Estrogen
|
Do you know the worth of estrogen? It is supplemented to fulfill the need of estrogen hormone in body.
It shows significant contribution in conception. It is given intramuscularly (IM) |
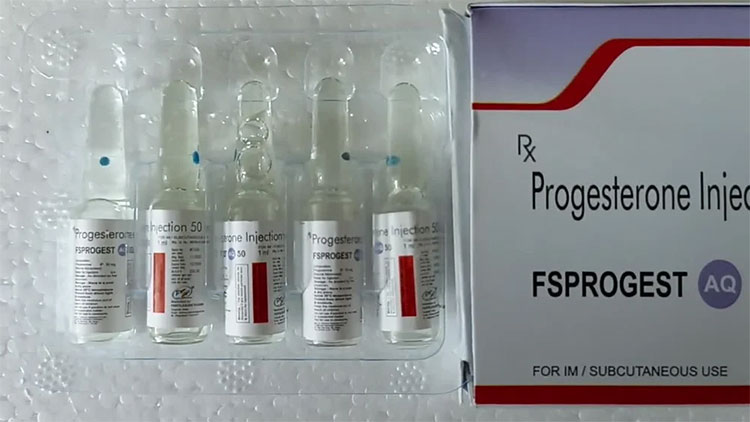
| Progesterone
|
Progesterone can be used to manage female reproductive system problems, like bring regularity in menstrual cycle.
This hormone can be given by subcutaneous (SC) or intramuscular (IM) route. |
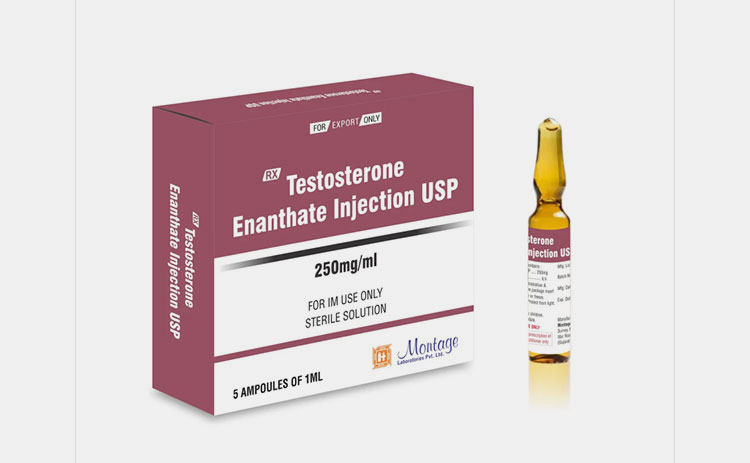
| Testosterone
|
It is known as an important male reproductive hormone. You can use it as replacement therapy or to treat hypogonadism.
Most of the time you can administer it by intramuscular (IM) route and sometimes subcutaneous (SC) route. |
| Human growth hormone
|
Lacking of growth hormone can cause problem in growth of children and adults.
Physician recommends supplementation of human growth hormone by subcutaneous or intramuscular route in case of severe deficiency. |
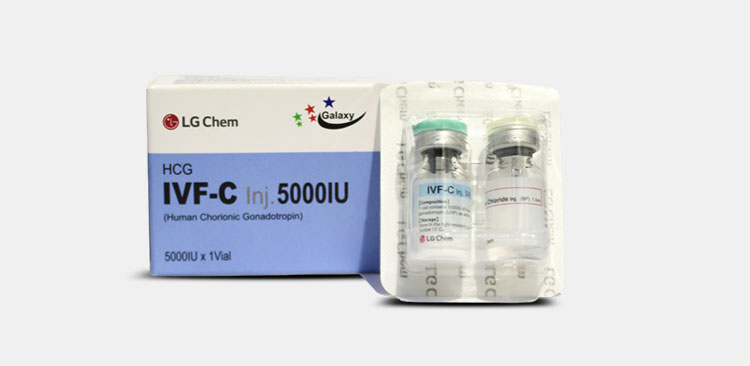
| Human chorionic gonadotropin
|
This hormone can be taken by those who are suffering from infertility issues or having low amount in body. You can give this hormone intramuscularly. |
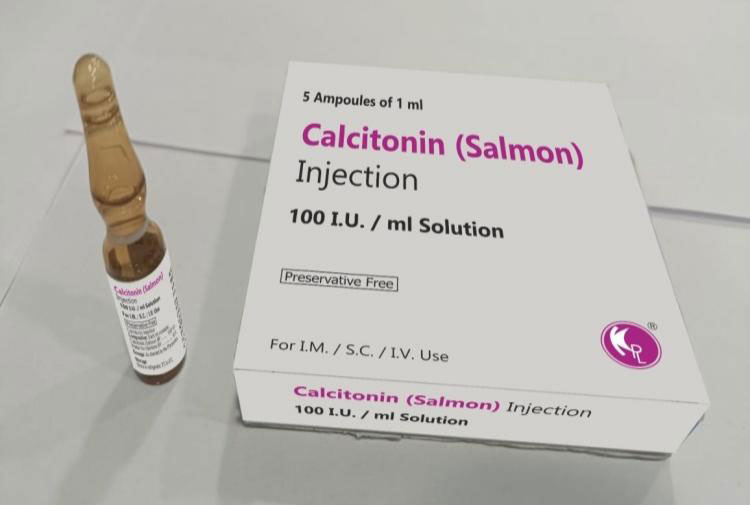
| Calcitonin
|
Have you heard about Paget’s disease? This disease belongs to bone, in which bones gets fragile and damaged. Calcitonin injection is used to treat this condition; other use is to administer intramuscularly to women having bone deformities due to osteoporosis. It can also help you when concentration of calcium gets increase in body. |
| Thyroid stimulating hormone
|
This hormone is used for diagnostic purpose. Are you known to thyroid cancer? This hormone is given by intramuscular route to confirm the levels of thyroglobulin in patients of thyroid cancer. |
| Glucagon
|
This hormone serves as a useful medication especially when there are low levels of sugar in body. You can give to diagnose health issues related to stomach and other organs. It can be administered by subcutaneous, intramuscular or intravenous route. |
5.What route of administration can be followed by products made under hormone manufacturing facility guidelines?
You are known to various routes that are effective for administration of hormones. Here you get to know details of different ways of administration.
| Routes of administration | Image |
| Intramuscular route:
You can introduce the hormone into body by injecting into muscles. This route offers you safe absorption of hormone without losing some amount by metabolism. Common intra muscular sites can be deltoid muscle present in upper arm or you can administer in hip or thigh for profound effects. |
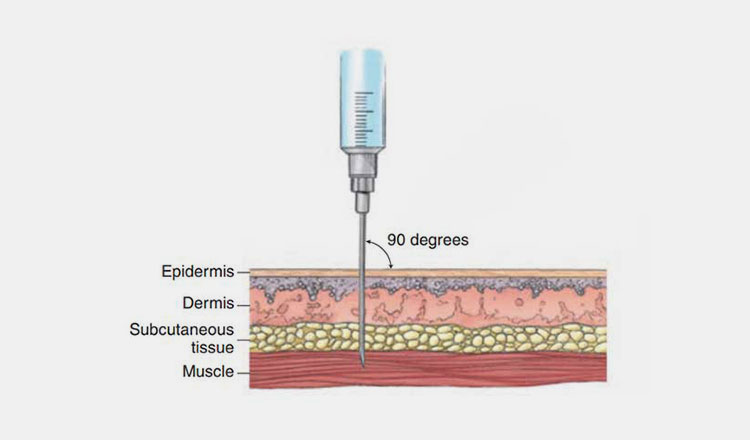
Picture Courtesy: Pharmapproach.com |
| Subcutaneous route:
This route provides you safe but sustained route to introduce medication in body. You inject drug in cutis layer of skin which is found beneath dermis and epidermis layer. |
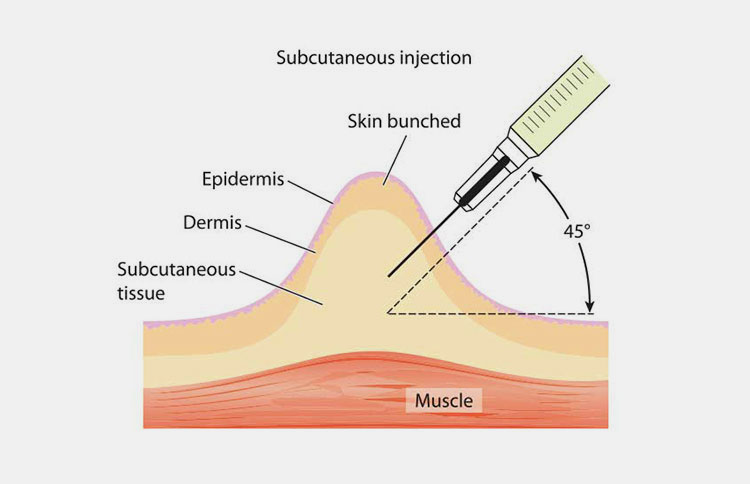
Picture Courtesy: Ausmed |
| Intravenous route:
This route works by introducing drug directly into blood vessel. You find it very effective and rapid way of administering drug into the body. |
Picture Courtesy: Active Plus Home Health Los Angeles |
6.What packaging containers are suitable according to hormone manufacturing facility guidelines?
Do you have idea that what containers are used to package hormones? This topic informs you about packaging containers that are supposed to be appropriate for hormone packaging according to hormone manufacturing facility guidelines.
| Packaging containers | Image |
| Vials: have you noted hormone injections are available in small cylinder shaped container? It is called as vial and it offer an appropriate way of dispensing hormone to keep it sterile and potent. Vial is made from borosilicate glass that is compatible with formulation. |  |
| Prefilled syringes: when it comes to administer a calculated dose without wasting time, prefilled syringe gives you best way. It is helpful in administering precise dose with great sterility and safety as prepared formulation is present in it. |  |
| Ampoules: when you have intention to administer single dose then ampoules presents you better choice. Hormone is filled in small glass made ampoule which keeps formulation sterile and uncontaminated. |  |
7.How products can be made according to hormone manufacturing facility guidelines?
Production Of Hormones According To Hormone Manufacturing Facility Guidelines-Picture Courtesy: Deposit Photos
Before starting the process of manufacturing hormones, always inspect about the facility where this manufacturing is carried out. You must be sure that process is following good manufacturing practices (GMP) and there is segregated place for every step. The environment provided for manufacturing must be maintained aseptic and controlled by utilizing different means.
| Steps of making hormone products | Information |
| Sourcing of raw material: | Have you thought from where you can get hormones? These are taken from plants and animals tissues and also by performing some chemical methods.
You must keep in mind that sourcing of raw material either its active ingredient or excipient, has great influence on quality of your final product, so always take this raw material from a reliable and recorded source. Raw material must be of high-grade quality. You must handle these hormones with care to avoid any toxicity. Sterile water is sourced by using water treatment system. |
| Mixing all ingredients: | Mixing of ingredients is an important topic and you have to perform it accurately.
You must note the thing that hormone is need to be dissolved in a sterile vehicle which has capability to dissolve it well. Like you can dissolve hydrophilic hormone in sterile water for injection or hydrophobic in lipid soluble solvent. After dissolving the hormone, you have to add other ingredients like stabilizer, buffer, or preservative) and mix it uniformly. Some formulations are suspensions or emulsions so they need to be homogenized well by using equipment like homogenizer, to form a uniform mixture. |
| Filtration: | Now you have to perform filtration by using sterile filters to remove impurities and microbes. |
| Filling and sealing; | Filling and sealing process has core importance and it requires specially designed equipment.
Aseptic filling lines are the best to fill and seal hormones in desirable dispensing containers like ampoules, prefilled syringes or vials. These filling lines are designed in such a way that supports the filling and sealing of specific container. You can observe that the machine follows the process by washing and sterilizing the containers, then filling it precisely and after that sealing it well to avoid contamination. |
| Lyophilization: | You may observe that some formulations are sensitive enough that they require lyophilization. Do you know what lyophilization is?
Lyophilization is a process in which you remove water content to convert it into powder. This process starts when formulation is made frozen, then pressure is applied to remove water. After that formulation changes to powder directly from frozen form. You can call this powder as freeze-dried powder. |
| Sterilization: | In order to keep your product sterile and safe, you perform the step of terminal sterilization. Autoclaves are used to perform this step. |
| Quality control: | You have to secure the quality of product, for this you have to perform some tests to confirm that this product attains quality according to standards.
These tests may include potency test, purity test, sterility test, etc. |
| Labeling and packaging: | After confirming about the quality, you may put forward the dispensing containers for labeling by the help of labeling machine and packaging into boxes by the help of cartoning machine. |
| Documentation: | You have to record and document important information related to every batch to avoid problem in future.
Such information may include traceability of ingredients, operator logs or maintenance log etc. |
8.What kinds of machines are required as per hormone manufacturing facility guidelines?
Have you any idea about the machines that take part in manufacturing of hormones? Important thing is that you have to ensure that the machines must meet the regulatory standards before starting any process.
| Machines | Function |
| Water treatment system:
AIPAK Water Treatment System |
This water treatment system helps you to sterile water for injection.
This sterile water for injection has high purity and is considered as best sterile vehicle for most of the hormones. |
| Mixing tank:
|
Mixing tank takes part in uniform mixing of ingredients. It has stirrer and temperature controlling system that helps you to perform the mixing process without any problem. |
| Homogenizer:
|
Some formulations that are thick in consistency require special equipment like homogenizers. These homogenizers help you to incorporate all the ingredients evenly. |
| Sterile filter:
|
Removal of impurities and microorganisms from formulation is made easy for you by using sterile filters.
This equipment uses 0.22 micron membrane to perform this process. |
| Vial filling line for liquids:
AIPAK Vial Filling Line For Liquids |
Vial filling line for liquids offers you suitable way to fill and seal hormones in vials in aseptic conditions. Restricted access barrier (RABS) is used to prevent risk of contamination. This system is used for all filling lines.
This vial filling line composed of four important parts, that can be: washing machine, sterilization machine, filling and stoppering machine, and capping machine. After filling with precise dose, vials are stoppered with rubber and capped by using aluminum or plastic caps. |
| Vial filling line for lyophilized powder:
AIPAK Vial Filling Line For Lyophilized Powder |
Vial filling line for lyophilized powder makes it convenient for you to fill and seal those formulations that are subjected for lyophilization after sealing in vials.
This equipment has divided into washing unit, sterilization unit, filling and stoppering unit and sealing unit. Vials are stoppered by using rubber and sealed by aluminum or plastic caps. |
| Lyophilizer:
|
Lyophilizer has great importance as it can change the state of formulation and reason is well known to you.
Formulations that lack the ability to remain stable in liquid form during storage conditions are changed to freeze dried form. Lyophilizer performs the process of lyophilization and makes it easy for you to store hormone product in a stable form. |
| Prefilled syringe production line:
AIPAK Prefilled Syringe Production Line |
Prefilled syringe production line has role in accurately fill and seal hormone in prefilled syringes.
It follows the process in a way that syringe is unpacked and then torn to get filled with hormone and after filling, plugging of syringe is carried out to make it air tight and avoid any contamination. |
| Ampoule filling line:
AIPAK Ampoule Filling Line |
Ampoule filling line is considered as an appropriate way to fill and seal hormone in ampoules.
It consists of four machines that are washes, sterilizer, filling and sealing machine. Sealing of ampoules is performed by using heat. Top part of ampoule is melted by using flame that helps in creating seal.
|
| Autoclave:
AIPAK Autoclave |
Autoclave has great importance in hormone manufacturing. Final sterilization of product is done by using autoclaves. Steam is used to sterilize the formulation.
|
| Labeling machine:
AIPAK Labeling Machine |
Labeling machine has the function to apply the printed labels on dispensing containers.
Adhesive method is used to apply the labels. Labels contain important information like product name, dose, route of administration, batch number etc.
|
| Cartoning machine:
AIPAK Cartoning Machine |
Cartoning machine has great part in securing the hormone product stability and potency during storage.
Hormones are packed in boxes, either single or multiple compartments to keep it safe during storage and distribution. |
9.What regulatory bodies are necessary according to hormone manufacturing facility guidelines?
Are you aware of the importance of regulatory bodies in manufacturing of hormone? These regulatory bodies can be international or national but they secure vital place in maintaining the process according to mandatory standards. National regulatory bodies work in their country and control local manufacturing. They proposed rules and regulations that aid you to produce a safe, sterile and potent product.
| Regulatory bodies | Information |
| WHO (world health organization)
|
WHO is an international organization that presents you guidelines for hormone manufacturing. These guidelines are known as good manufacturing practices (GMP). |
| ISO (international organization for standardization)
|
This organization gives guidelines about the working of machines and systems. |
| FDA (food and drug administration)
|
FDA is a national regulatory body of USA, which ensures the quality manufacturing of hormones or other biologics. |
| DEA (drug enforcement administration)
|
This organization is also USA based and your hormone manufacturing facility must meet standards proposed by DEA. |
| EMA (European medicines agency)
|
This organization works in Europe and every hormone manufacturing facility must be compliant with EMA. |
| NMPA(national medical products administration)
|
In china, NMPA is responsible for controlling manufacturing of hormones. So your manufacturing facility must be competent to standards told by NMPA. |
10.What can be the ways to maintain compliance with hormone manufacturing facility guidelines?

Ways To Maintain Compliance With Hormone Manufacturing Facility Guidelines-Picture Courtesy: ShineMD
You have learnt about how hormones can be manufactured according to hormones manufacturing facility guidelines. Have you wondered that there should be some ways to maintain the compliance with these guidelines? This topic is narrated to make you aware about all these ways and this will be helpful for you to get the understanding about importance of maintaining the compliance.
| Ways for maintenance | Information |
| Employment of good manufacturing practices (GMP): | You must look for compliance of your manufacturing facility with good manufacturing practices. These guidelines can be proposed by international (WHO, ISO) or national (FDA, EMA, NMPA etc.) regulatory bodies. |
| Preserving the manufacturing area: | There are some specifications for manufacturing facility that are needed to be fulfilled, like
You must make sure that every step must be carried out in separate area. You must check about the clean rooms as manufacturing of hormones require clean rooms that have controlled environment. You must use all means to keep the environment aseptic and controlled like HVAC system, RABS (restricted access barrier system), etc. You can assess the environment of manufacturing facility by taking samples and testing it. Machines must be inspected and calibrated according to mandatory standards. |
| Regular cleaning and decontamination: | You must carry out the cleaning and decontamination process accurately and regularly.
You must use suitable cleaning agents for cleaning purpose. Machines can be cleaned or sterilized by using clean in place or sterilize in place systems. Machines can be inspected by performing swabbing test, in which you swab and rinse a sample to check the hygiene standards. |
| Well-trained working staff: | You must train the working staff excellently to avoid accidents.
You must always allow trained staff in hormone manufacturing premises. |
| Implementation of CAPA: | Do you know the meaning of CAPA? This means corrective and preventive action. This is a system which is built to address the problems that can be happened during manufacturing and how can you prevent them?
You must implement CAPA in manufacturing facility to bring improvement in manufacturing facility of hormones. |
| Documentation: | You must always keep an eye about documenting important information of batch. This brings you ease in pointing any problem related to that batch manufacturing. |
Conclusion:
Hormone manufacturing facility guidelines ensure the sterility and efficiency of manufacturing of hormone. Guidelines are fully compliant with good manufacturing practices (GMP). You cannot get a high quality product until and unless you follow these guidelines. Maintaining these guidelines in manufacturing facility is another important thing to pursue safe, sterile and potent hormone product. If you still feel the need of gaining more information about hormone manufacturing facility guidelines, you can visit AIPAK ENGINEERING at any time!
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours