Cholera Vaccine Production: The Complete FAQ Guide In 2025
Have your ears ever been struck by news of deaths caused by cholera? Basically, cholera is an acute diarrheal illness and it is happened by taking food or water which is contaminated by bacterium vibrio cholera. According to World Health Organization (WHO), globally last year 1.3 to 4 million cases of cholera have been reported and 21000 to 143000 people were died from this hazardous disease. These figures reflect the need of quality cholera vaccine production for good global health.
This guide will help you to get understanding about complete process of cholera vaccine production, its composition, equipment used for production and also the packaging container utilized for its dispensing. It is necessary for you to get mindful of the importance of this vaccine in order to bring awareness among other people and supporting the health system.
1.What do you mean by cholera vaccine production?
Cholera Vaccine Production
Cholera vaccine production relates to the whole procedure involved in making a vaccine which is used to combat cholera, a threatening diarrheal disease caused by bacterium vibrio cholera. Severe dehydration occurs due to continuous diarrhea which leads to death of the person if not treated properly. Vaccination aids in preventing spread of disease and saves people.
Cholera vaccine production is crucial in keeping society protected from cholera. As a preventive measure, this vaccine can be consumed by people traveling to cholera-endemic places, residents in high-risk areas or people during outbreaks or humanitarian catastrophe. In general, these vaccines are quite tolerated and can cause temperate side effects like stomach cramps or nausea.
2.Do you know the privileges cholera vaccine production brings to the society?
To understand the necessity of any vaccine production, you always need to learn that what this production is giving to your society. This topic will make it convenient for you to understand that how this cholera vaccine production is advantageous to keep the society healthy and growing!
Role in prevention of a life-threatening disease:
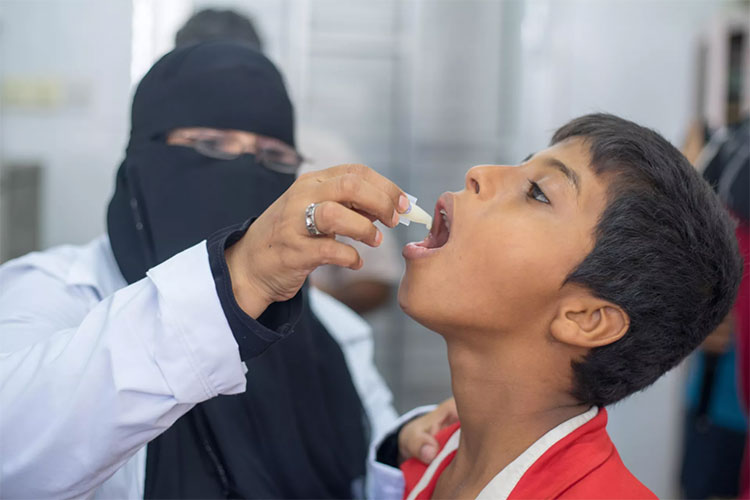
Cholera Vaccine Product Prevents Life-Threatening Disease Of Cholera-Picture Courtesy: UNICEF
Cholera is a life-threatening disease and it creates deficiency of water and minerals by causing severe diarrhea which ultimately leads to loss of life. Water and minerals have chief role in settling the homeostasis of body and their inadequate amount can cause death. This cholera vaccine production helps in reducing the chances of disease and death.
Controls outburst of disease:

Cholera Outbreaks Can Hit Refugee Camps
It seems common to see outbreaks of cholera in case of natural disasters or humanitarian crises as the system of sanitation & hygiene is shattered. Vaccinations camps are built to keep people save from this disease and in this way cholera vaccine production brings people to life by controlling the surge of disease.
Shields highly-threat population:
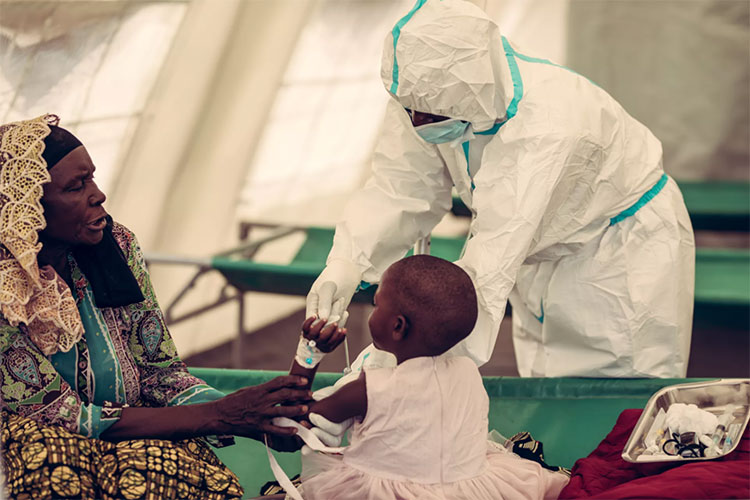
Cholera Vaccine Production Shields Children From Getting Affected By Cholera
There are so many people in the world, forced to live with low quality system of water sanitation & hygiene. Most common reason is their dwarfish socio-economic state. In this situation cholera vaccine production shields the low immunity people like children, old aged individuals, sick ones or pregnant ladies from getting this disease.
Lowers burden of healthcare system:
Cholera Vaccine Production Lowers The Burden Of Healthcare System-Picture Courtesy: UN News
The cholera vaccine production supports good health of population otherwise it becomes really hectic and unbearable when a vast number of people come with same degree of disease.
3.What are the different vaccines produced by cholera vaccine production?
Now days, three different cholera vaccines are created by cholera vaccine production and all are given orally. These vaccines work by producing antibodies against bacterial parts and toxin of bacterium vibrio cholerae. Below are the details of three available cholera vaccines.
Dukoral:
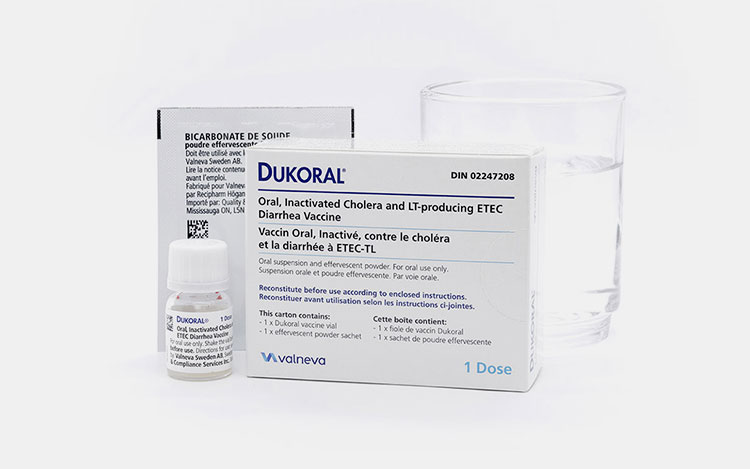
Oral Cholera Vaccine Dukoral
It is the first ever developed oral cholera vaccine which is inactivated, that means it contains killed cells of bacteria and recombinant cholera toxin.
A buffer solution is essential to be taken with Dukoral in order to keep it safe from effects of gastric acid.
This vaccine can be administered to individuals of two years of age or more than it.
It needs two full doses to show its profound effects. Three doses are administered in children between 2-5 years old.
The optimum gap between 2 doses can be at least 7 days and at most 6 weeks. These two doses provide shield against cholera disease for two years.
This vaccine was mainly developed with the intention to be used by travelers going to affected places.
Shancol & euvichol:
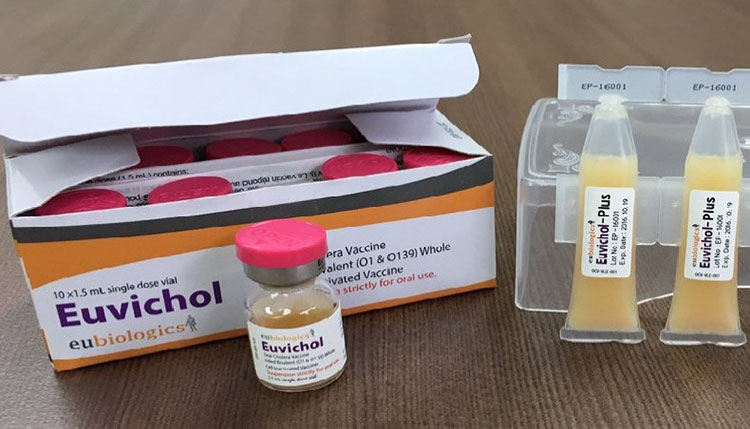
Oral Cholera Vaccine Euvichol
These are two variant cholera vaccines, created by same manufacturer. These are inactivated vaccines and comprised of different strains of killed vibrio cholerae.
These vaccines don’t have necessity to be administered with buffer solution.
Two complete doses are given to create enough effects against cholera and can be administered to one year old child or more than it.
At least a two week gap between two doses is favorable.
Dual doses of these vaccines grant protection against cholera for three years.
A short duration protection can also be achieved by administering a single dose of this vaccine.
4.What is the history of cholera vaccine production?

First Oral Cholera Vaccine Was Made In 1980s In Sweden By The Name Of Dukoral-Picture Courtesy: Devex
Cholera vaccine has always been demanded as cholera threat was there to haunt people for losing their life. Till the 1970s, injectable cholera vaccines were produced and administered. A breakthrough was achieved in 1980s, when first effective oral cholera vaccine production had occurred and it was developed in Sweden in the name of Dukoral. In the 1990s, oral cholera vaccine was presented to use against cholera disease.
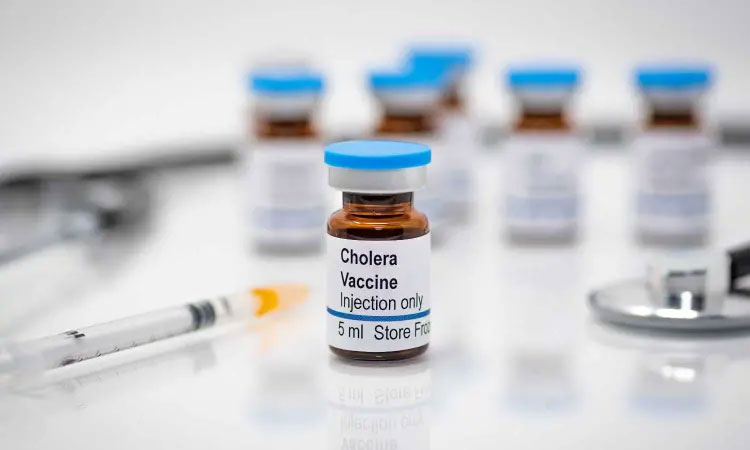
Injectable Cholera Vaccine Was Used Till 1970s-Picture Courtesy: Medical Dialogues
In 2013, another oral cholera vaccine production had taken place in South Korea by the name of Euvichol plus. In 2016, live and attenuated oral cholera vaccine production had happened, which was certified for travelers by US FDA.
Live Attenuated Oral Cholera Vaccine-Picture Courtesy: Paul-Ehrlich-Institut
Since then oral vaccine production is dominating the market due to high effectivity and low side effects. Recently, in 2024, Euvichol S-vaccine was made which is advanced version of Euvichol-plus. It is cost effective and potent and will be helpful to solve the problem related to shortage of cholera vaccine.
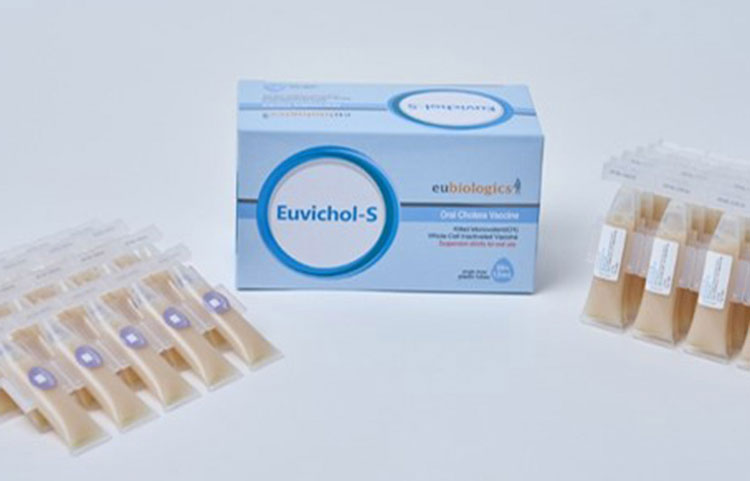
Euvichol-S Oral Cholera Vaccine-Picture Courtesy: BioWorld
5.Can you elaborate about the components utilized in cholera vaccine production?
When you began to know about the cholera vaccine production, a question hits your mind what are the components of this vaccine? As components of cholera vaccine have their role to fight with this deadly disease. Components are elaborated below:
Strains of bacteria:
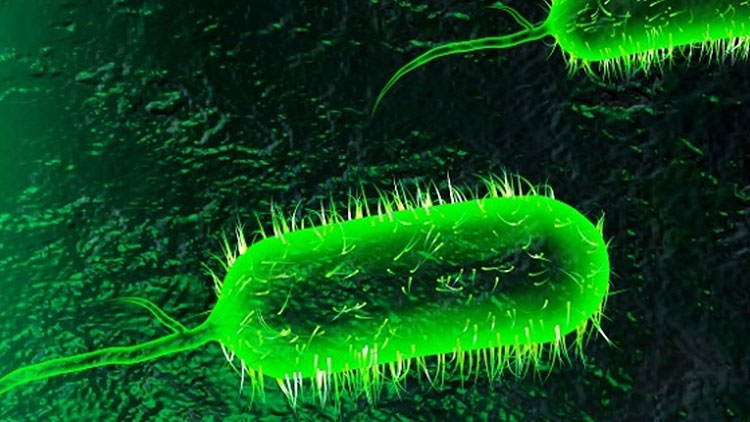
Bacterial Strain Of Vibrio Cholerae-Picture Courtesy: Harvard Gazette
Two kinds of bacterial strains are intended to use in production of cholera vaccine production.
- Inactivated
- Live attenuated
Most common practice tells us that inactivated or killed bacterial strains are used extensively. Serogroups that are used in cholera vaccine production are vibrio cholera O1 or O139 because these are the main serotypes responsible for causing cholera disease. Process is very simple, firstly bacteria are cultured, harvested then made inactivated by killing with heat or certain chemicals. Another type is live attenuated bacterial strain, this kind of bacterial strain is genetically changed into harmless one but have tendency to induce immunity.
Cholera toxin B subunit:
Cholera Toxin B Subunit-Picture Courtesy: Biotium
Vaccines like Dukoral have cholera toxin B subunit as their component. It is recombinant and non-toxic part of cholera toxin. It plays key role in inducing a brilliant immune response.
Buffers & stabilizers:
Bicarbonate buffer:
Bicarbonate Buffer
This buffer is taken along with Dukoral vaccine in order to keep it safe against gastric acid.
Stabilizers:

Sodium Citrate Is Used As Stabilizer-Picture Courtesy: Ingreland
These are incorporated with vaccine to prolong its shelf life by keeping it stable. Commonly used stabilizers are sodium citrate, magnesium chloride or amino acids.
Preservatives:
Thimerosal Is Used As Preservative-Picture Courtesy: Medline
Multi-dose formulation of vaccine may have chances to contain preservatives like thimerosal in it. But now days, preservatives are not included.
6.What packaging options can be used in cholera vaccine production?
Packaging material plays an important role in maintaining sterility, stability and efficacy of vaccine. So here it is very important for you to know about the packaging material used in cholera vaccine production.
Single dose glass vials:
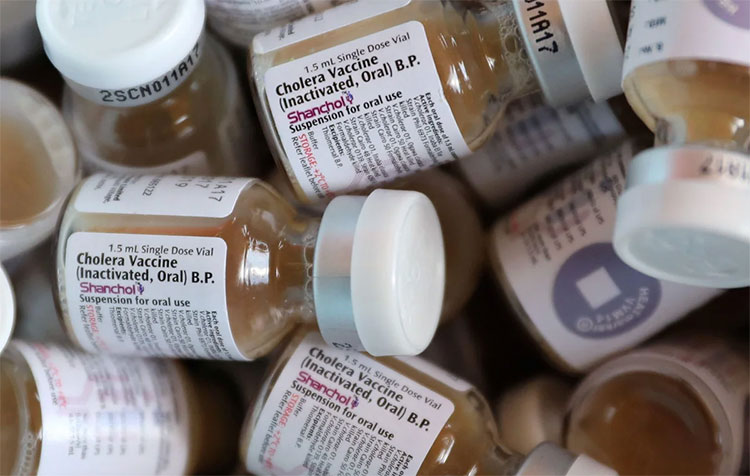
Single Dose Vials Of Oral Cholera Vaccine-Picture Courtesy: The Gaurdian
Most common type of packaging material used to dispense cholera vaccine is glass vial. It is made from type 1 borosilicate glass, which is an appropriate choice to dispense liquid sterile products. This packaging material is also chemically resistant, ensuring unreactive storage of vaccine. Usually Dukoral and Shancol are dispensed in this glass vials which are sealed by inserting rubber stopper and crimping aluminum caps over it.
Plastic ampoules:

Oral Vaccine Packaged In Plastic Ampoules-Picture Courtesy: Wikimedia Commons
Plastic ampoules are also used to dispense cholera vaccine. These ampoules are good in keeping vaccine sterile and stable. These are made from high-density polyethylene (HDPE) or low-density polyethylene (LDPE). This kind of packaging is favored for huge population immunization program or places contain fewer resources. These have several benefits including light in weight so easy to carry, un-breakable and provide convenient way to administer oral dose. Euvichol is packaged in plastic ampoules.
7.What method is followed by cholera vaccine production?
Process for cholera vaccine production is simple and basic, that is common used for developing a vaccine. There are steps that involved in efficient cholera vaccine production, discussed below:
Bacterial strain selection:
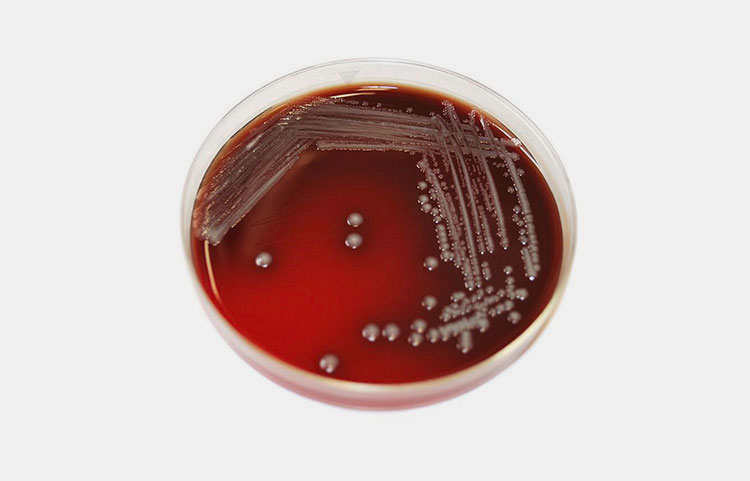
Growing Vibrio Cholerae-Picture Courtesy: Reddit
Bacterial strain is needed to develop vaccine. Mostly vibrio cholerae two serogroups are reason of causing cholera disease i.e. O1 and O139. So the strains of these two serogroups are chosen to induce the immune response.
Inactivation or attenuation of bacterial strain:
Inactivation Or Attenuation Of Vibrio Cholerae-Picture Courtesy: Creative Biolabs
Inactivation of bacterial strain is carried out by following two steps. Firstly you grow bacteria in culture medium. Bioreactors are used to provide optimum conditions for growth. After that bacteria are killed by applying heat or a chemical like formalin.
Attenuation is achieved by making genetically modified strains,that are harmless but have ability to trier immune response.
Purification:

Sterile Filtration-Picture Courtesy: Corning
Bacterial cells or components like cholera toxin B subunit are purified from impurities or toxins by using ultrafiltration or sterile filters.
Formulation:

Formulation Of Cholera Vaccine-Picture Courtesy: Pure Source
The inactivated or attenuated bacterial strain is incorporated with stabilizers or preservatives to form cholera vaccine. The mixing of ingredients is carried out in mixing tank.
Quality control:

Vaccine Testing-Picture Courtesy: CEPI
Tests are performed to inspect the sterility, potency, safety and uniformity of vaccine. Clinical trials can also be performed to ensure its safety in humans.
Filling & packaging:
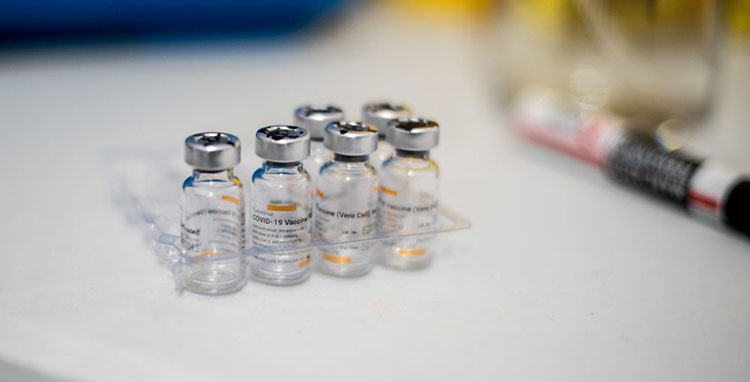
Glass Vials Containing Cholera Vaccine-Picture Courtesy: Money Web
The finalized product is dispensed in glass vials or squeeze tubes for oral administration. Buffer is packed in sachets. All products are stored under optimum conditions to keep stability intact.
8.What are the machines used for cholera vaccine production?
Efficient equipment is required for cholera vaccine production. Aseptic condition is the first priority in sterile products manufacturing, which is also maintained in cholera vaccine production.
Bioreactors:

Bioreactors-Picture Courtesy: Hamilton Company
Bioreactors are used on industrial basis to grow vibrio cholerae under the influence of favorable conditions. It is a vessel which keeps pH, temperature, oxygen and nutrients in suitable range and support cultivation of bacteria.
Centrifuges:

Centrifuge-Picture Courtesy: Labkafe
Bacterial cells are cultivated in culture medium, so it becomes necessary to separate these bacterial cells from culture for making vaccine. Centrifuges are used to isolate bacterial cells from culture medium.
Mixing tank:

Mixing Vessel
Mixing vessel is crucial for proper mixing of components used to make vaccine. Bacterial strain (inactivated /attenuated), stabilizers or preservatives are blend homogenously by the help of mixing vessel.
Liquid vial filling line:

Aipak Liquid Vial Filling Line
This machine offers precise filling, stoppering and capping of liquid formulation in to vials. Whole process in performed under aseptic conditions and restricted access barrier system (RABS) is used to minimize the contamination risk. It has inspection system to avoid any visual defect in finalized product.
Autoclaves:

Autoclave-Picture Courtesy: MRC-Laboratory Equipment
Sterilization is the basic requirement of cholera vaccine production and autoclaves are used to facilitate the sterilization of media and equipment.
Labeling machine:

Labeling Machine For Cholera Vaccine Production-Picture Courtesy: Pharmaceutical Labelling Machine Manufacturer
Labeling machine is referred to automated packaging equipment used in cholera vaccine production to apply labels on vials or ampoules of cholera vaccine. Printed labels are applied on cholera vaccine that contains important information including batch number, expiration etc.
Cartoning machine:

AIPAK Cartoning Machine
Cartoning machine is used to insert the final product into boxes or cartons. Usually tuck-in flaps are used to close the boxes of cholera vaccines.
9.How to ensure the quality and safety of cholera vaccine production?

Compliance To GMP Is Integral To Ensure Quality And Safety Of Cholera Vaccine Production-Picture Courtesy: MIAS Pharma
You can easily understand the need of cholera vaccine production, but it becomes equally important to ensure the quality and safety of it. An effective and stable vaccine can only makes difference by showing powerful effects against cholera.
Compliance with good manufacturing practices (GMP) is integral to make the process safe and effective. There are certain standards described by WHO and other regulatory bodies like FDA, must be practiced during each step of production including sanitation, documentation and personnel training.
Safe & Effective Cholera Vaccine-Picture Courtesy: DW
10.What are the important aspects of cholera vaccine production?

Important Aspects Of Cholera Vaccine Production-Picture Courtesy: International Vaccine Institute
Cholera vaccine production has some important aspects which should be considered in order to achieve a high quality cholera vaccine, which is safe and effective at the same time. This topic provides you awareness regarding important aspects of cholera vaccine production.
| Important aspects | Why you should consider them? |
| Recognizing the pathogen: | Here it is very important to know the causative agent of cholera, and then you can produce vaccine of high efficacy. As you know two serogroups have been proven to cause the cholera disease (O1 and O139). Structure acknowledgement is also helpful in making effective vaccine. |
| Vaccine type: | Inactivated vaccines were generally produced from the beginning. Few years before attenuated vaccines are also introduced in market. You have choice of selecting the type of vaccine you need to produce as both has different production steps, stability and ability to boost immune response. |
| Selection of antigen: | Antigen means the bacterial strain which causes the induction of immune response. In this regard, whole bacterial component is utilized or sometimes cholerae toxin subunit B (CTB) is also used in vaccine. So reason is very simple for using CTB in vaccine, which is to protect intestinal mucosa from harmful effects of bacterial toxin. |
| Process of production: | Process of production must be carried by using efficient machines. Aseptic conditions should be maintained. Follow good manufacturing practice (GMP) guidelines to ensure safe and stable product. |
| Quality control: | Quality control is the core of the production. If final product is not up to the mandatory standards, whole process is considered low quality. So sterility, stability and potency testing is very much important to be carried out. |
| Regulatory compliance:
|
Competency with regulatory standards is integral. Guidelines provided by WHO or other national regulatory body is necessary. Prequalification by WHO is required for use of vaccine worldwide. |
| Storage conditions:
|
Temperature should be maintained 2-8 C to keep vaccine stable. Cold chain management must be good to maintain the safety and efficacy of vaccine. Insulated vaccine transport kits with ice packs can be used to store cholera vaccine. Light and moisture exposure must be avoided to save vaccine from degradation. Also don’t freeze the vaccine, just refrigerate it. |
11.What could be the expiration of vaccine made by cholera vaccine production?
When Cholera Vaccine Gets Expired? –Picture Courtesy: UNICEF
Like any other vaccine, cholera vaccine also gets expired. Expired vaccine loses its stability and efficacy so you need to be considerate about the expiration of vaccine. Under optimistic storage conditions, oral cholera vaccines have shelf life of 2-3 years from date of manufacturing.
There are no highlighted signs of expiration but you can encounter some visual changes in formulation of vaccine that shows its expiration, like:
- Cloudiness or color variation in liquid.
- Particles are floating or settled at the bottom of liquid.
- Damaged vial like having crack, leakage or corrosive cap.
12.What provocations can be faced during cholera vaccine production?
Cholera vaccine production is simple and yields good quality product but it does has some challenges that need to be discussed to offer understanding of them.
Requirement of cold chain:

Cold Chain Requirement For Cholera Vaccine Storage-Picture Courtesy: Wikipedia
It becomes really challenging to store vaccines in remote or low resourced places as they need cold storage to be in stable form.
Production capacity:

Cholera Outbreaks Demand For Increase Cholera Vaccine Production-Picture Courtesy: WHO
Cholera vaccine production has limited capacity to manufacture vaccines. In hours of need like during some humanitarian crisis or outbreaks, demand increases many folds which become a challenge to compensate the shortage.
High cost of development:

High Investment Is Required For Cholera Vaccine Production-Picture Courtesy: Vecteezy
Effective and long-lasting cholera vaccine demands for high investment, which creates a challenging situation for manufacturers.
Regulatory hurdles:

Prequalification From WHO Is Required To Use Vaccine Globally-Picture Courtesy: Pew Research Center
It is mandatory for cholera vaccines to match regulatory standards. This feature demands for rigorous testing and approval which creates a challenging situation.
Poor health infrastructure:

Poor Sanitation And Hygiene-Picture Courtesy: UNICEF
Cholera usually affects places that have low socio-economic conditions. Fewer incentives for vaccine development and poor water & hygiene system make it hard to meet the requirement of society regarding vaccine availability.
Conclusion:
Cholera vaccine production secures an integral part in maintaining control and prevention of cholera disease. Oral cholera vaccine production had made progress in fighting with this life-threatening disease in vulnerable and endemic areas but few challenges are still there. Increasing production capacity, providing cold chain for storage and maintaining regulatory standards can enhance the production in much more ways. Are you seeking a reliable source for getting more information? AIPAK engineering is solution to your finding, you can contact us anytime!
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours

