Antibody Manufacturing: The Complete FAQ Guide in 2025
Ever wondered how life-saving antibodies are actually manufactured? In today’s time, antibody manufacturing has become the most essential part of biotechnology, as it allows the development of highly effective treatments, especially for immune-related diseases.
Antibody manufacturing is a crucial process that plays a key part not only in treatments but also in diagnostics and research. Many companies have focused on manufacturing antibodies due to the reason of its role in the development of life-saving medicines for treatments of cancer, autoimmune diseases, and infections. In this comprehensive guide, we will learn about the fundamentals of antibody manufacturing. Let’s get started.
1.What is antibody manufacturing?
Monoclonal antibodies- Picture courtesy: MD Anderson Cancer Center
The process of antibody manufacturing involves the creation of antibodies in a laboratory or industry. Antibodies are special protein that plays a key role in detecting and fighting off harmful bacteria, viruses or toxins.
Scientists grow antibodies by culturing special cells into a nutrient-rich formula, enabling them to grow and produce antibodies on a larger scale. The antibodies produced in the antibody manufacturing process are used in monoclonal treatment of cancer, COVID-19 rapid tests, research experiments, and autoimmune disease treatments.
2.Why is antibody manufacturing important?
Antibody Manufacturing- Picture courtesy: Creative BIOLAB
The importance of antibody manufacturing is described below:
| Description | Application |
| Advanced Approach in Treatments | Antibodies are widely used to treat autoimmune diseases, cancer, and viral infections. These monoclonal antibodies are manufactured to specifically focus on disease cells providing efficient treatment with minimal side effects. |
| Rapid Response to Global Outbreaks | After COVID-19, antibody manufacturing expanded significantly due to its role in diagnostic testing and treatments. It facilitated in creation of a rapid test system for the coronavirus. |
| Business Growth and Investment | The global market for antibody manufacturing is growing steadily and will continue to expand further. This growth is supported by pharmaceutical and biotechnology industries for antibody research and innovation. |
| Customized Medications | Personalized antibody manufacturing is carried out according to a specific patient's treatment needs or disease type. This advancement in modern medicine is achieved due to continuous innovation in antibody manufacturing. |
| Research Growth | Antibody manufacturing plays a key role in the field of medical research and innovation. This includes studying for detection and treatments of emerging health issues and improving the healthcare standard globally. |
3.In which field is antibody manufacturing commonly used?
The applications of antibody manufacturing are explained below:
Medical Intervention
Monoclonal Antibodies Handling Cancer Cells- Picture courtesy: STEMCELL TECHNOLOGY
Antibody manufacturing plays a key role in therapies for different kinds of diseases. In conditions such as cancer monoclonal antibodies detect and treat cancer cells, preventing further growth of tumor. Autoimmune conditions like rheumatoid arthritis or lupus are cured by antibodies by block parts of the immune system from attacking tissues.
Diagnostics Fields

Antibody testing for diagnostics- Picture courtesy: News Medical.net
Antibody manufacturing is crucial for diagnostic test because of their ability to precisely bind to target antigens in several diagnoses. These include rapid testing: For COVID-19, HIV or dengue. The antibodies quickly detect the presence of these pathogens in the body rapidly. Antibody manufacturing is also essential for cancer diagnosis; it helps detect the formation of abnormal cells in blood or cells.
Biotechnology Industry

Pesticide Analysis- Picture courtesy: SMS Labs
Besides medicine, antibody manufacturing plays an important role in industrial applications and biotechnology. For instance, in bioprocessing antibody manufacturing plays a key role, since it helps in the purification of certain proteins during the process of drug manufacturing.
4.What are the common types used in antibody manufacturing?
The common types formed by antibody manufacturing are:
Monoclonal Antibodies

Monoclonal Antibody- Picture Courtesy: Kingmans Regional
Monoclonal antibodies also called mAbs are lab-manufactured antibodies. While natural antibodies form a diverse group, monoclonal antibodies are all identical and target one specific kind of pathogen such as a virus, bacteria, or cancer cell. The monoclonal antibody is manufactured by genetic engineering technology in CHO cells and the fusion of two types of cells such as B cells and myeloma cells, resulting in hybridoma.
Polyclonal Antibodies
Polyclonal Antibody Manufacturing- Picture courtesy: Eurogenetec
Polyclonal antibodies (pAbs) are a group of different antibodies manufactured by several B cells. As opposed to monoclonal antibodies, they attach to several antigens. They are usually made by injecting an animal and acquiring antibody serum from its blood.
Polyclonal antibodies generally play a key role in research experiments and diagnostic purposes. The process of polyclonal antibody manufacturing involves the immunization of animals, after immunization response blood is collected for serum preparation. The serum goes through a purification step to collect purified antibodies.
Recombinant Antibodies
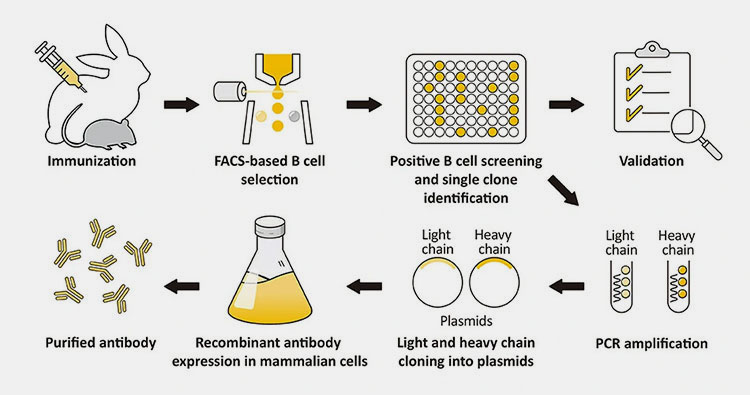
Recombinant Antibody- Picture Courtesy: GeneTex
Recombinant antibodies are lab-made antibodies, using the method of genetic engineering method. A small piece of DNA is taken; the scientist then performs gene identification. The gene is inserted into CHO cells with the method of genetic engineering to produce antibodies. The process of recombinant antibody manufacturing involves the identification of genes. These genes are incorporated into expression vectors, and after that cell culturing is performed to gather antibodies.
5.What steps are involved in antibody manufacturing?
Antibody manufacturing- Picture courtesy: Labcorp
Antibody manufacturing is a multi-step procedure involving several efficient methods. The step by step process of antibody manufacturing is mentioned below:
| Steps | Description |
| Antigen Preparation | In this step, the target antigen is purified by removing impurities to ensure its stability. |
| Immunization Formation | The purified antigen is combined with a specific protein to produce an immunogen that can extract an immune response from host cells. |
| Immunization | An animal is immunized with the immunogen to start antibody manufacturing. |
| Isolation of cells | After immunization, the B cells are isolated for fusion with myeloma cells, which then create hybridoma cells that are capable of antibody production. |
| Cell Culture | The hybridoma cells formed are cloned and monitored for antibody manufacturing. These cloned cells are cultivated in bioreactors to generate antibodies on a large scale. |
| Antibody Purification | Using the chromatography method, the produced antibodies are purified to ensure precise product and stability. |
| Formulation | Following purification, the antibodies manufactured are converted into a final product preparing them for therapeutic use. |
| Quality Control | The finalized product is tested for quality control and regulatory compliance to ensure safety, sterility, and stability. |
| Packing and Storage | Finally, the antibodies are sealed into containers or vials under aseptic conditions and stored in a regulated environment for effectiveness. |
6.What types of machines are used in antibody manufacturing?
The antibody manufacturing process is a complex process involving a number of specialized equipment to ensure stability. The entire process is outlined and illustrated through a flowchart below.
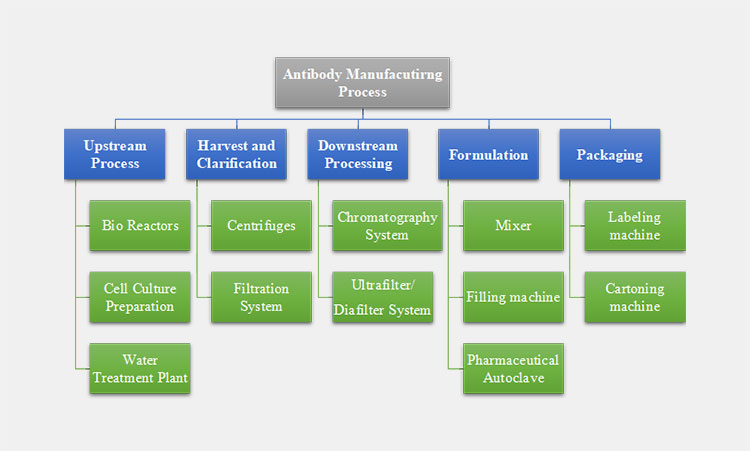
Flowchart of Antibody Manufacturing Process
Upstream Process
The upstream process is the initial process of antibody manufacturing. This means how to inoculate the cells into the host cell for cultivation. The process machines are included as:
Bioreactor Machine

Bioreactor
The process includes bioreactors, which are specific kinds of vessels made of stainless steel for reuse or disposable material for one-time use ranging from 5 to 10 liters to up to 20,000 liters for small scale or large-scale production depending on the requirement.
Biosafety Cabinet for Cell Culturing

Biosafety Cabinet for Cell Culturing- Picture courtesy: HK- Hope
Cell culture media preparation system helps this process by sterilizing nutrient mixture to support safe cell growth. For this purpose, you need to use a biosafety cabinet that offers maximum protection from external unwanted particles and microbes to interfere with sensitive procedures. It is installed with a HEPA filter and utilizes positive pressure with laminar air flow thus inside air quality is sterile and ideal for cells and personal safety.
Water Treatment Plant

AIPAK Engineering Purified Water Treatment System
For antibiotic manufacturing cell culturing, media preparation and related sensitive steps need water involvement. Remember you can’t use untreated water. It must be sterile and pure. That’s why water treatment plant installment is essential. It is associated with the installment of either reverse osmosis unit, distillation plant, deionization plant, etc. The following units help in the removal of unwanted ions, hard water elements, the presence of microorganisms, etc. Subsequently, the water used is pure and suitable for antibody manufacturing.
Harvest and Clarification Equipment
Harvesting and clarification equipment in antibody manufacturing is used to purify the solution from cell culture. The machines involved in this process are:
Centrifuge Machine

Centrifuge Machine- Picture courtesy: LabGear Australia
To separate the cells from the mixture, a centrifuge machine is used. Here, various centrifuge tubes are filled with mixture and set to a particular RPM or high-speed revolution for a certain time period. As a result, the particular cells are settled down leaving plasma above. You can easily remove unwanted layers and get the required substances.
Filtration Machine

Antibody filtration machine- Picture courtesy: filstep
This is an essential unit for almost all healthcare and research processes. In antibody manufacturing, the filtration machine helps in the filtration of foreign particles protein content, and debris. The machine is ideal for the purification of solution that is heat-sensitive in nature. Therefore, for safety and elimination of microbial chances, the filtration unit is significantly utilized.
Downstream Processing
The downstream processing involved the estimation and purification of the antibody solution. The process includes the following machines:
Chromatography System

Chromatography Machine- Picture courtesy: Fisher Scientific
Once the cells are produced with the help of cultivating or culturing, they are passed through the above steps such as filtration and separation. The chromatography machine is used for further analysis and fractionation of content present in the achieved formulation. In this machine, you can eliminate the extent of impurities present on the basis of polarities. In every analytical process, the chromatography machine is used for purification and identification of element present in the solutions.
Ultrafiltration System

Ultrafiltration System- Picture courtesy: ALFA LAVAL
To ensure the formulation is cleared of unwanted ions and molecules, the ultrafiltration unit is required. In this process, the small ions or molecules are filtrated by using the semi-permeable membrane filtration system.
Formulation
During the formulation of antibodies, a certain set of machines are used.
High Shear Mixer

AIPAK High Shear Mixer Granulator
The antibody solution is basically composed of water, concentrated granules, buffer, preservatives, and trace elements. The final formulation and dilution need a proper mixing to merge these substances appropriately. For this purpose, a high-shear mixer is used that ensures shear forces for the dispersion of particles and allows antibody solutions to homogenize thoroughly without affecting their effectiveness.
Filling Machine
The antibody manufacturing final formulation is either filled in vials or bottles. The types of machines used for this purpose are:
Aseptic Liquid Vial Production Line

AIPAK Engineering Aseptic Liquid Vial Production Line
The aseptic liquid vial production line is an automatic and advanced innovation where the formulation is accurately dispensed in vials. The production line is composed of a set of machines including with washing machine that utilizes ultrasonic waves to wash the walls of vials and removes the debris from the edges if present.
The sterilization tunnel helps in the decaying and killing of microbes and purifies the vials with the help of high temperatures. The vial filling machine takes and fills the particular volume of antibody formulation. The stoppering unit now encloses the vials by hooking the stopper tightly.
Pre-Filled Syringe Production Line

AIPAK Engineering Pre-Filled Syringe Production Line
The highly sterile solution for filling of antibody is a prefilled syringe production line that helps you in the unwrapping, filling, and plunging of syringes. The production line is highly recommended for antibody manufacturing with small volume dispensing ranging from 0.1ml to 20ml. The filling line is also suitable for vaccination and related processing due to its modern structure and promising features.
Pharmaceutical Autoclaving Machine

AIPAK Engineering Pharmaceutical Autoclave
Once all filling and sealing of packaging material is done. For further sterility pharmaceutical autoclave machines are used. The work of this machine is based on temperature, induced pressure, and vacuum for certain times. By implementation of these three parameters can result in deactivation and degradation of microbial progression and presence.
Packaging
No product can be marketed without its packaging. The antibody manufacturing process’s last steps are based on packaging procedures and include the following machines:
Labeling Machine

AIPAK Labeling Machine
To name the brand, information related to antibodies, when it was manufactured, how to reconstitute, how to store, and relevant information is mentioned on the labeling. The labeling machine is used for the application of labels over packaging materials. There are semi-automatic and automatic machines that are mainly integrated with the production line to bring essential tasks in a seamless way.
Cartoning Machine

AIPAK Cartoning Machine
The cartoning machine is used to form cartons, placement of antibodies products inside, and secure them. There are various innovative machines available where automatic leaflet insertion, error detection, etc. are featured for quality results.
7.What aseptic techniques are essential in antibody manufacturing?

Aseptic Techniques in Biotechnology- Picture Courtesy: News- Medical
Adapting aseptic techniques while antibody manufacturing is essential in order to focus on effective and stable manufacturing. These techniques help prevent microbial contamination. Key points considered in aseptic techniques are as follows:
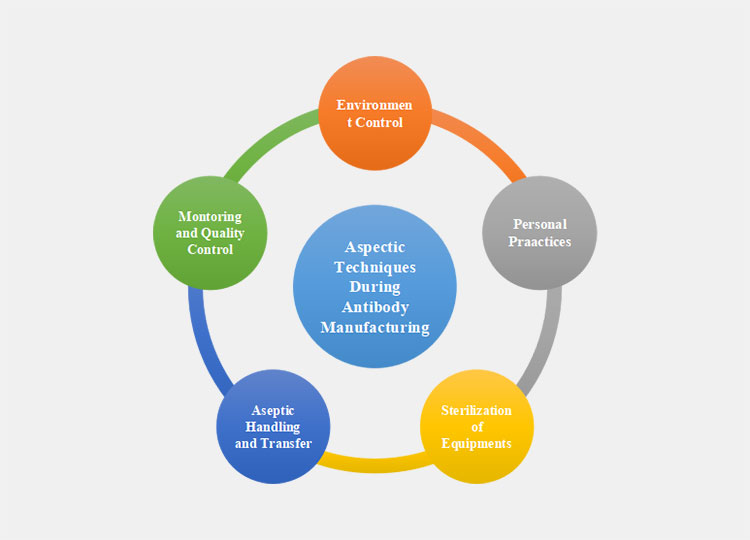
Key Points For Aseptic Antibody Manufacturing
Environment Control

Aseptic Techniques for Antibody Manufacturing- Picture Courtesy: American Medical Compliance
Antibody manufacturing is done in sterilized environments such as Grade A (ISO 5) to prevent contamination. HEPA Filtration is used to maintain sterilized air quality followed by adding positive pressure to prevent contamination from outside environments.
Personal Practices

Antibody Storage- Picture Courtesy: Single Use Support
Personal sterile practices include manufacturers wearing sterile gloves, gowns, masks, hair cover, and goggles to minimize contamination. Proper training of manufacturers on aseptic techniques and minimized movement to prevent interaction with critical areas should be carried out to ensure a safe environment for antibody manufacturing.
Sterilization of Equipment

Protective Covering for Antibody Manufacturing- Picture Courtesy: Germ free
Sterilized equipment for antibody manufacturing is essential. There are several different techniques used to sterile equipment such as autoclaving in which the equipment is sterilized using autoclaves, and filtration solutions containing 0.22 µm filters are used to purify solutions.
Aseptic Handling and Transfer

Antibody Manufacturing Procedure- Picture Courtesy: Global Grand Challenges
Hygienic handling of antibody manufacturing equipment and careful transfer methods are carried out in order to prevent the solution and product from being contaminated from skin flora and outside microbes. These techniques are vital to ensure the stability and efficiency of antibody manufacturing.
Monitoring and Quality Control

Aseptic Techniques during Antibody Manufacturing- Picture Courtesy: AGC Biologics
To maintain quality control and compliance with regulatory requirements, continuous testing of the final product is carried out to ensure sterility. Final products are tested for endotoxins and other contaminates that can be caused by microbial contamination like pyrogens and allergens.
8.What is the storage procedure after antibody manufacturing?

Storing antibodies- Picture courtesy: POCONO
After antibody manufacturing, their storage is important to maintain sterility and its functioning. The storing standards vary for both short-term antibodies or long-term antibodies. Below are the main storage procedures required for antibody manufacturing:
| Storage Form | Duration | Temperature | Description |
| Short-Term Antibody
(Liquid Form)
Cold storing practices for monoclonal antibodies- Picture courtesy: K2 Scientific |
Less than one month | 2-8ᴼC | Short-term antibody is stored in liquid form, mostly in the refrigerator at 4ᴼC.
It’s important to use an appropriate buffer like PBS or Tris. A buffer solution will prevent the antibody from becoming too acidic or basic. 0.02-0.05% sodium azide is added into the solution as a preservative to prevent antibodies from microbial contamination. |
| Long-Term Antibody Storage (Liquid Form)
Operating with antibodies- Picture courtesy: Bio-Rad Antibodies |
More than one month | -20ᴼC to -80ᴼC | For long term storage, the antibody should be stored at -20 to -80ᴼC.
For longer storage, it is essential to divide the antibody solution into smaller tubes. For instance, a 1 ml volume of antibody is to be divided into 10 small tubes. Each tube will be 100 microliters in volume. This process is called aliquot. Aliquoting is the process that mitigate the possibilities of contamination and minimizes freezing and thawing cycles. |
| Lyophilized (Freeze-Dried Form)
Lyophilized antibody vial- Picture courtesy: Immunostep |
Months to years | 2-8ᴼC
Room Temperature |
The lyophilized form of antibodies is most suitable for shipment and long-term storage as they remain dry and sealed.
It is important to add sterile water before using lyophilized antibody |
9.What is the duration of effectiveness after antibody manufacturing?
Effectiveness of antibody manufacturing: Picture courtesy: Boston Herald
The duration of effectiveness after antibody manufacturing depends on their form and storage conditions. The detailed breakdown is mentioned below:
Liquid Antibodies
Antibody Vial- Picture Courtesy: The Independent
Liquid antibodies are ideal for short-term use, a small amount of preservative sodium azide is added to prevent contamination. The effectiveness period is typically 2 weeks to 4 weeks.
Lyophilized (Freeze-Dried) Antibodies

Lyophilized Antibody- Picture courtesy: Pel-Freez Biologicals
Freeze-dried antibodies are stable for 2 to 10 years because of their dry and sealed form. The storage conditions for lyophilized antibodies are 2ᴼC to 8ᴼC at room temperature. They are required to add sterile water before usage. Once reformed, their stability depends on their storage conditions.
10.What kind of packaging is used for antibody manufacturing?

Antibody Manufacturing- Picture Courtesy: Proteintech
Packaging plays a key role in maintaining sterility and safety of antibodies ensuring effective treatments and diagnostics. Depending on the type and formulation, there are different kinds of packaging used for antibody manufacturing. Some of them are mentioned below
| Packaging | Description |
| Vials | Vials are small sterilized glass containers. For packaging of liquid formulations, these kinds of containers are generally used. For instance, injectable monoclonal antibodies |
| Prefilled Syringes | These are syringes that are filled prior with a precise dose of antibody-drug for injecting. This kind of packaging reduces preparation time, prevents the risk of contamination, and ensures precise dosing. |
| Bottles | Bottles for packaging are used for large amounts in antibody manufacturing. They keep the antibody formulation in large quantities and then it is divided into small portions like vials or syringes. |
11.What are some common challenges in antibody manufacturing?
| Common Challenges | Description | |
| Low Expression Levels | Antibody manufacturing in large amounts can be challenging especially if the antibody has a complex structure or is unstable. | 
Antibodies and Antigen- Picture courtesy: Technology Networks |
| Purification Challenges | It is very complicated to remove host cell proteins, DNA, endotoxins or clumps without compromising on antibody quality.
Protein A chromatography is the method used to purify antibodies but it also comes with its own challenges like being expensive and harsh on antibodies disrupting their quality. |
Antibodies in isolated Form-Picture courtesy: Dreamstime.com |
| Regulatory and Quality Control | Maintaining quality control during antibody manufacturing that is used especially in treatments is very challenging.
The process involves strict quality control, analytical testing and precise documentation requiring a lot of attention, time and resources. |
Invivo Antibody- Picture courtesy: Assay Genie |
| High Production Cost | Antibody manufacturing is an expensive procedure since it requires specific materials like purification resins and cell culture media.
To comply with regulatory requirements, customized raw materials and specific packaging is required. Such standards make antibody manufacturing costly. |
Monoclonal antibody- Picture courtesy: Contract Pharma |
| Contamination Risk | Antibody manufacturing is a complex process in which cross-contamination and microbial contamination can happen during manufacturing.
This contamination can make the antibody unsafe and ineffective to use. |

Sterile chromatography vial- Picture courtesy: Chromatography Toda |
12.What factors should be considered while opting for antibody manufacturing?

Antibody Manufacturing- Picture Courtesy: Gresco
| Factors | Description |
| Purpose of Antibody | Before starting antibody manufacturing, it is important to consider the purpose for which the antibody will be used.
There are different purposes for antibody manufacturing including research purposes, treatment, and diagnostics. |
| Type of Antibody | Different types of antibodies should be kept in mind before antibody manufacturing.
For instance, there are monoclonal antibodies ideal for treatments, Polyclonal for research purpose and recombinant for adaptability. |
| Target Antigen | The nature of the target antigen should be kept in mind before antibody manufacturing. The type of antigen determines what kind of antibody manufacturing method would be used.
Target antigen examples include proteins, peptide, or pathogens. |
| Production Scale | Before opting for antibody manufacturing, it is important to consider what quantity of manufacturing is required.
Laboratory usage requires small scale while commercial use requires large scale quantity. |
| Expert Support | Antibody manufacturing is a complex process; it is important to choose reliable support with a consistent result.
Specifically account for features like compliance with regulatory requirements like ISO and GMP. |
Conclusion
Antibody manufacturing is a very critical yet highly effective process that contributes not only to therapeutic but also to research purposes. The industry is likely to expand further in the future with innovations in healthcare. With this guide, we hope you have explored the vast knowledge of antibody manufacturing and why it is so important for healthcare. Are you interested in exploring equipment involved in antibody manufacturing? We AIPAK Engineering is a team of experts who strive best to bring innovative technology related to antibody manufacturing. You can trust our amazing products on a friendly budget. For more information, please head over to the AIPAK Engineering website.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours