CGRP Monoclonal Antibodies: The Complete FAQ Guide In 2025
Have you ever wondered if there is a smarter way to manage migraine other than using a usual pills? Imagine a solution that is made with high precision that relief you from migraine pain. Welcome to the world of CGRP monoclonal antibodies that is changing lives and challenging the old treatments.
In this FAQ guide, you will know how CGRP monoclonal antibodies work, the benefits, the risks, and the most frequently asked questions in 2025 about these antibodies. Whether you are migraine sufferer, the manufacturer of these CGRP monoclonal antibodies or the caregiver, then this guide is worth reading. The answers might surprise you. So, let’s begin!
1.What do you know about CGRP monoclonal antibodies?
CGRP monoclonal antibodies-Picture courtesy: virtualheadachespecialist.com
CGRP monoclonal antibodies (mAbs) are biologic drugs that are specifically designed to treat the migraine. Monoclonal antibodies are specific proteins or peptide chain.
CGRP monoclonal antibodies are proteins that inhibit the secretion of chemical known as calcitonin gene-related peptide (CGRP) and its receptors. CGRP are neuropeptide that is found in brain and when the CGRP mAbs binds to CGRP or block the CGRP receptors, then the patient may not feel migraine.
2.Do you know how the CGRP monoclonal antibodies work in your body?

Working of CGRP monoclonal antibodies against migraine
Have you ever wondered how the CGRP monoclonal antibodies sets off the migraine mysteriously? Here is detailed explanation of how actually these CGRP monoclonal antibodies works in your body when you take it.
When you feel migraine, your brain nerves activated and release the chemical it’s due to the chemical known as calcitonin gene-related peptide. This leads to transmission of pain attack in your nervous system, and you feels migraine. To block the pain receptors, there are two mechanisms.
Some types of CGRP monoclonal antibodies like fremanezumab blocks the CGRP molecules. Do you know how it works? These antibodies attack on CGRP molecules and prevents them from binding to receptors on nerve cells. The nerve cells may not detect the stimuli and hence, you may not feel migraine.
Some CGRP monoclonal antibodies like erenumab directly attacked on CGRP receptors, stopping them from working.
3.Enlist the types of CGRP monoclonal antibodies?
Have you ever heard about the types of migraine antibodies? Well, there are four types of CGRP monoclonal antibodies. These types are fall into two categories and that categories are divided based on the target mechanism. So, let’s explore these types:
CGRP receptor targeting antibodies:
Erenumab (Aimovig ®):
Erenumab (Aimovig ®)-Picture courtesy: migrainewarriors.org
The target of erenumab is CGRP receptors. It is the only antibodies that act upon the CGRP receptor instead of CGRP or you can say peptide itself. You already knows that CGRP get activates and leads to migraine but the erenumab occupy the receptors and prevent the CGRP from activating. The CGRP does not receive any stimuli and so, the pain receptors does not generate.
It is injected into the body subcutaneously once in a month.
CGRP receptor targeting antibodies are usually available in 70 mg and 140 mg prefilled auto injectors.
CGRP ligand targeting receptors:
There are three types of CGRP ligand targeting receptors. These are:
- Fremanezumab
- Galcanezumab
- Eptinezumab
Fremanezumab ( Ajovy ®)
Fremanezumab (Ajovy ®)-Picture courtesy: medic.co.il
The target of Fremanezumab is CGRP ligand. These monoclonal antibodies directly attached to the CGRP. So, the pain signals do not reach to the CGRP receptors. Do you know the vasodilation occur and that create a sense of pain. But here, the CGRP receptors do not activates and hence, stops the pain signaling in the brain. These antibodies are usually injected by those people who prefers less frequent injections.
You can inject it subcutaneously.
These monoclonal antibodies are available in two options for dosing. One can injected once in a month and the other can be injected in every 3 months.
Galcanezumab ( emgality)
Galcanezumab ( emgality)-Picture courtesy:medscape.com
It is also the type of CGRP ligand targeting antibodies. These monoclonal antibodies are suitable for those patients that suffer from episodic cluster headache because they have faster onset of actions. These monoclonal antibodies binds to the CGRP and prevents it from interacting with receptors and hence, stops the pain signaling.
You are inject it through subcutaneous route.
You can inject these monoclonal antibodies once in a month.
Eptinezumab ( Vyepti®)
Eptinezumab (Vyepti®)-Picture courtesy: goodrx.com
This injection is suitable for those patients that prefers to visit the clinic quarterly. The injected antibodies binds to the CGRP protein and avoid the stimuli from reaching to the CGRP receptors. In this way, the signaling to the brain stops and person may not experience migraine.
It is unique CGRP monoclonal antibodies because it is the only type that can be injected intravenously.
It takes about 30 minutes to infuse.
4.What are the benefits of using CGRP monoclonal antibodies?

CGRP monoclonal antibodies reduces the severity of the migraine-Picture courtesy: orthopedicsandpain.com
If you are suffering from frequent and chronic migraine, then the use of CGRP monoclonal antibodies provides you several benefits. This is new technology to treat the migraine. The benefits are as follows:
| Benefits | Description |
| Targeted migraine prevention | The CGRP monoclonal antibodies are used to block the CGRP which is calcitonin gene- related peptide that plays an important role in the creation of your migraine pain. |
| Reduces frequency, severity and duration | If you are suffering from frequent migraine e.g. more than 15 days per month. Then, by using CGRP mAbs the number of days of migraine suffering is reduced. Some people gain more benefits from using it, they have milder headache, shorter migraine attacks and can improve the normal life style. |
| Long lasting effects with convenient dosing | Are you fed off from the daily intake of pain killers? You don’t need to worry because these monoclonal antibodies can relief you from migraine if injected once in a month or quarterly. |
| Can be used alongside acute migraine treatments | Are you using your usual migraine medications like triptans? You can use CGRP monoclonal antibodies alongside other medications because these antibodies are preventive, not abortive. |
| For patients who failed other preventive medications | Have you used older medications like propranolol for migraine treatment? But your immune system did not respond to lower the migraine then this is the best option for treatment of migraine. |
| Easy administration | You can easily administered the CGRP monoclonal antibodies to your body at home because they are available as auto injectors just like insulin pen. If you wants to inject it under doctor supervision, then Eptinezumab is available which can be quarterly administered in clinics. |
5.Do you know the potential risks of CGRP monoclonal antibodies?
Any risks or side effects of CGRP monoclonal antibodies?
If you are suffering from other health issues, then you should clearly know about the side effects of using CGRP monoclonal antibodies. Or some people can well tolerate it but some may suffer from side effects or risks. These are as follows:
| Risks or side effects | Description |
| Injection side reactions | When you inject the CGRP monoclonal antibodies to your body, then you might noticed the redness, swelling, or itching at the injection site. |
| Constipation | The risk of constipation is high especially when erenumab injected into your body. Sometimes, the dietary changes can helps you from get rid of constipation. |
| Allergic reactions | Some people immune system is very sensitive so, if you inject the mAbs in to your body and you feels rashes, hives and itching then this may be due to allergic reactions caused by CGRP monoclonal antibodies. |
| Not studied well in pregnancy and breastfeeding | After research and studied about the usage of CGRP monoclonal antibodies in pregnant or breastfeeding woman, it is usually not recommended for them. But still the research is going on for proper effect but avoid these monoclonal antibodies in such condition may prevent you from bad consequences. |
| Immunogenicity risk | When you inject CGRP monoclonal antibodies into your body, the body immune system may prepare antibodies against this biologic drugs and might reduce its effectiveness. Although it is very rare but can happens. |
| Risk for people with certain conditions | If you are patient of severe cardiovascular disease, or chromic constipation then you must be cautious while using CGRP monoclonal antibodies. |
6.How to manufacture the CGRP monoclonal antibodies?
The step-by-step preparation procedure for the manufacturing of CGRP monoclonal antibodies required high controlled and sterilized environment. You should carry every step with great precautions. Here is a detailed manufacturing procedure that should be followed:
Cell line development and cell banking:
Gene cloning:
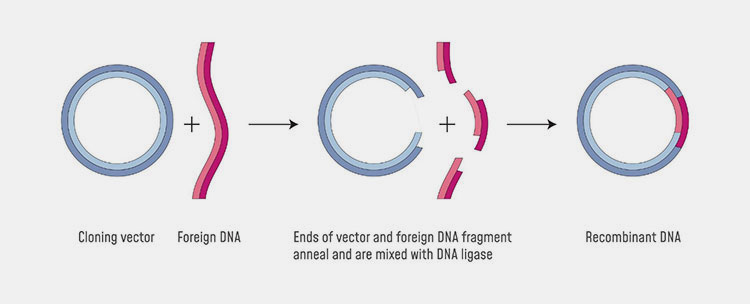
Gene cloning-Picture courtesy: news-medical.net
In the DNA, gene sequencing occurs where different codes for different traits reside. If you are scientist or researcher, you should isolate the gene sequence where the codes for CGRP monoclonal antibodies reside. You should isolate that gene and should be inserted into the plasmid vector. The gene sequence will get integrated into the plasmid vector. You should insert this into the host cells where monoclonal antibodies will produced.
Stable cell line selection:
The transferred cells are grown and then screened to find out the best clones for functional antibodies. You should select the highest quality antibodies clones and then should be expanded. The expanded clones are then stored as master cell bank and working cell bank under very low temperature.
Upstream processing: cell culture and antibody expression:
Seed train expansion:
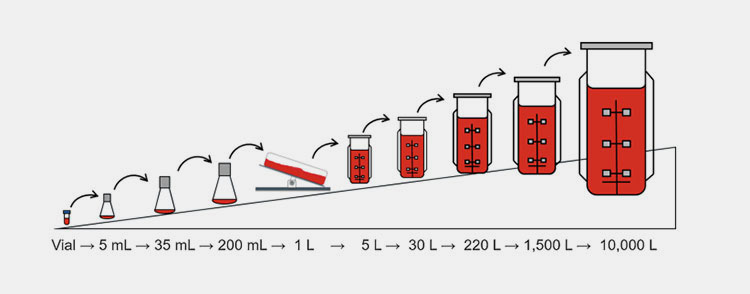
Seed train expansion-Picture courtesy: link.springer.com
The cells present in cell banks are then cultured in bioreactor to gradually expand in size. The cultured cells are then shifted to larger bioreactor where the cell culture scaled from milliliters to liters.
Production culture:
The stainless steel bioreactor where sterile nutrient-rich media reside, at controlled temperature and PH, the cells are cultured. Over 14 days, the cells secrete CGRP monoclonal antibodies into the culture media.
Harvesting and clarification:
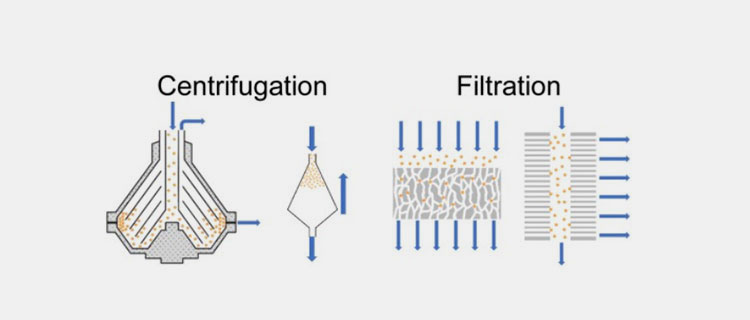
Centrifugation and filtration of CGRP monoclonal antibodies
Harvesting:
After the production phase, the cell culture fluid is collected. The cells and debris is separated by using centrifugation and microfiltration.
Clarified harvest:
After the microfiltration, you will collect the fluid solution that contains CGRP monoclonal antibodies. This solution is now ready for purification.
Downstream processing:
Purification:
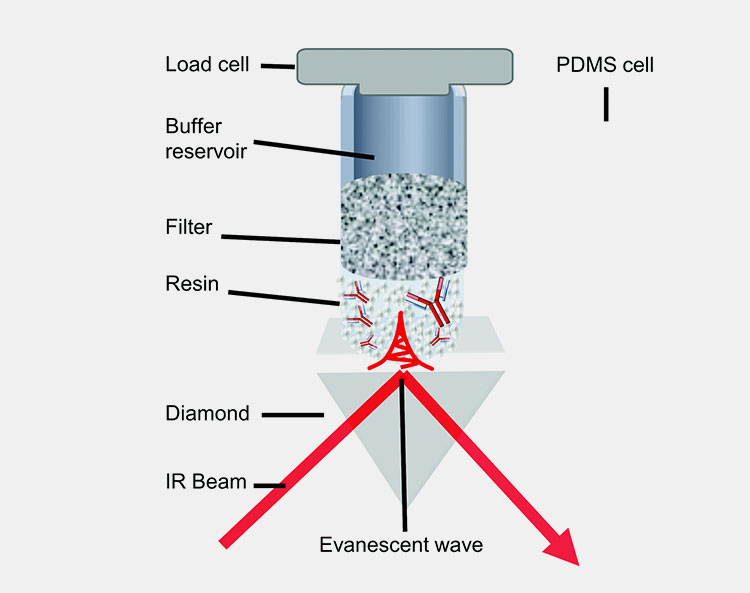
Purification of CGRP monoclonal antibodies
In this step, pure antibodies are obtained. You should use a resin where CGRP monoclonal antibodies stick to it and impurities are washed away. The sticked antibodies are then washed away by using special buffer solution. The antibodies are then processed through chromatography where impurities are removed. You will get a final formulation of antibodies.
Formulation:
The purified antibodies obtained are then mixed with other ingredients like stabilizers and excipients. The stabilizer mostly used is sucrose and excipients should be histidine buffer. In order to prevent the aggregation in antibodies, you should mix polysorbate 80. After mixing, the final formulated product is then passed through 0.22 micron filters to remove microbes etc. after that, the solution should be filled in the desired packaging.
Filling and packaging:
Filling of CGRP monoclonal antibodies into the vials
The sterile product solution is then filled into sterilized syringes that are pre-filled, auto-injector pens or vials depending on the product types. The filled products are then labeled and packaged into the boxes. The products should then be stored at low temperature.
Quality control tests:
Each batch of CGRP monoclonal antibodies should be tests through HPLC technique, sterility tests, and various other tests to ensure the sterility, purity and shelf life of the finished solution.
7.What are the machines involved in the manufacturing of CGRP monoclonal antibodies?
The main machines involved in the manufacturing of CGRP monoclonal antibodies should be sterilized and pharmaceutical grade. The flowchart for such machines are as follows:
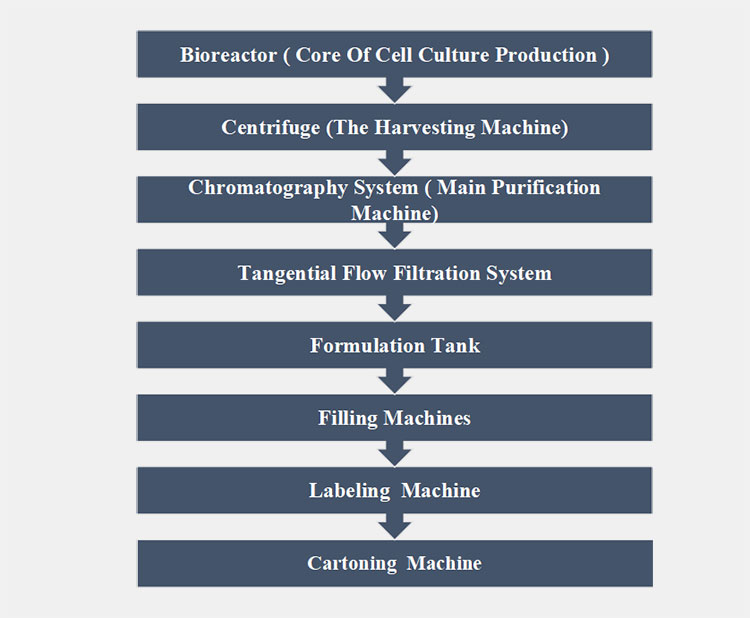
Bioreactor ( core of cell culture production )

Bioreactor-Picture courtesy: maxongroup.com
Bioreactor is a stainless steel tank where cultivation of cells generally Chinese hamster ovary cell (CHO) cells are cultivated that secretes the desired CGRP monoclonal antibodies. Nutrients are feed into the reactor which is essential for the growth of antibodies rich cells. Do you know the bioreactor has also automatic sensors? But what kind of sensors? The bioreactor is equipped with automated sensors for temperature, PH, dissolved oxygen and agitation speed. In this tank, the antibodies culture is obtained.
Centrifuge (the harvesting machine)

Centrifuge-Picture courtesy: analis.com
The monoclonal antibodies rich solution may also contain debris. For the separation of CGRP monoclonal antibodies rich cells and debris, centrifuges are used. For small scale harvesting, you can use batch centrifuge. If you wants to clarify the batch in a continuous flow, you can use disk stack centrifuges. These centrifuges are made up of stainless steel which ensures the sterility. The function of centrifuge is to separate the cells from impurities.
Chromatography system ( main purification machine)

Chromatography system-Picture courtesy: biotage.com
The CGRP monoclonal antibodies solution is then passed through chromatography system. The chromatography system has automated software control and multi-column setups. The main function of chromatography is to separate the monoclonal antibodies from proteins, DNA and other proteins. There are various types of chromatography. Each one has a specific function.
You can use protein A affinity for the capturing of monoclonal antibodies.
Ion exchange chromatography can be used for the removal of charged impurities.
Tangential flow filtration system
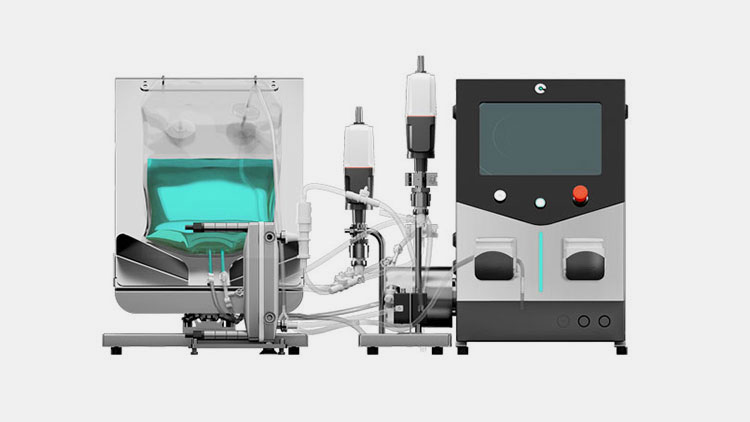
Tangential Flow Filtration System-Picture courtesy: pro-analytics
The purified antibody solution is passed through the tangential flow filtration system where the purified antibody solution is concentrated. This system has filter membrane through which the fluid solution flows tangentially. The tangential flow reduce the clogging. From solution the continuous separation of antibodies occur. This system automatically control the flow of liquid, pressure through which the fluid flows and the volume of the flow-able liquid. In this way, the precise and accurate separation of CGRP monoclonal antibodies occur.
Formulation tank

Formulation tank
Now, it’s time for the final drug formulation. You should add the purified CGRP monoclonal antibodies along with other excipients and stabilizers. The excipients and stabilizer are sucrose, polysorbate 80, and histidine buffer. These all are mixed in stainless steel tank which ensures the sterility of the final drug. It has magnetic stirrer or impellers in which all the ingredients are mixed well. This tank provides high sterility to the product because it is designed having a capability of clean-in-place (CIP) and steam-in-place (SIP). You can easily clean and steam the tank after each batch.
Filling machines
The CGRP monoclonal antibodies are available in vials as well as in pre-filled syringes. So, the machines used for filling in the vials and pre-filled syringes are as follows:
Vial liquid production line:

AIPAK engineering vial liquid filling machine
The vial liquid production line is composed of vial washing machine, dryer sterilization, filling stoppering machine, and capping machine. It can handle various sized vials, automatically washed and clean the vials, dry them, filled each vial with a desired volume of CGRP monoclonal antibodies, cap and seal the individual vial. Each step is done in a high sterilized environment. All the machines meet with the GMP standards and hence, suitable for the filling and sealing of the sensitive products like monoclonal antibodies.
Pre-filled syringes production line:

AIPAK engineering pre-filled syringes production line
The above pre-filled syringes production line can automatically unpack, tear, fill, and plugging of disposable syringes and packages the monoclonal antibodies into it. All the machines in this production line are accordance with the GMP regulation. It can fill the syringes from 0.1 ml to 20 ml. The CGRP monoclonal antibodies are filled into the syringes by mean of metal pump, ceramic pump or via peristaltic pump. You can customized the relevant products according to the specific demands.
Labeling machine:

AIPAK labeling machine
The filled vials and pre-filled syringes are then transferred to the labeling machine. In the labeling machine, each vial and pre-filled syringe is labeled with the drug name, dosage, how to use it, expiry date, batch number and serialization occur. This ensures the safety of the product. The serialization and tractability of these products allow to meet the standards of global pharmaceutical regulations.
Cartoning machine:

AIPAK cartoning machine
The packed vials and pre-filled syringes are then packed into the secondary cartons. The cartoning machine automatically picks the flat cartons, erect them into the boxes, place the vials and pre-filled syringes into it, folds the flaps and seal the box. In this way, the vials and pre-filled syringes are safe and ready for storage. The products are cartons should be stored in cold rooms (2◦C to 8◦C).
Quality control analytical instruments:
Different instruments are used to check the purity, safety and stability of the CGRP monoclonal antibodies. They are as follows:
ELISA reader:

ELISA reader-Picture courtesy: hercuvan.com
ELISA is enzyme linked immunosorbent assay. This technique is used for confirming the activity, concentration and purity of the CGRP monoclonal antibodies. It ensures that the monoclonal antibody correctly binds to the CGRP target. It can also confirm the impurities, if impurities found then those are removed during downstream steps.
HPLC:

High Performance liquid chromatography (HPLC)
The HPLC test is used to detect the purity and integrity of the CGRP monoclonal antibodies. It detects the impurities present in the product. It can be also used for the detection of shelf-life of the products.
Mass spectrometer:

Mass spectrometer
Mass spectrometry is used for the detection of sequence of the peptides in CGRP. It can also detect the molecular mass of the CGRP monoclonal antibodies. In this way, it can detect the purity and potency of the CGRP monoclonal antibodies.
8.What are the storage conditions for the effectiveness of CGRP monoclonal antibodies?

2-8-degree-celsius-freezer, laboratory refrigerator-Picture courtesy: beinginternational.com
The CGRP monoclonal antibodies should be stored at proper temperature and conditions for the safety, effectiveness and sterility of the product. So, below are the proper conditions to store the CGRP monoclonal antibodies.
| Storage conditions | Description |
| Recommended storage temperature | You should store the CGRP monoclonal antibodies at 2°C-8°C. At this temperature, the structural stability and biological activity of the monoclonal antibodies are ensured.
At this refrigerator temperature, the protein degradation and denaturation is prevented. |
| Do not freeze | You should never freeze the monoclonal antibodies or you can say below 0°C. If accidentally freezes, the protein irreversible denaturation occur and hence, peptides precipitation occurs. |
| Protect from light | Do you know the sunlight contains UV light? When the UV light exposed to CGRP monoclonal antibodies, the peptide bond get denatured and hence, the effectiveness of the monoclonal antibodies reduced. So, always keep the antibodies containing liquid products in packaging. |
| Avoid high temperature | It is recommended to not store the monoclonal antibodies at very high temperature (above 25°C). If exposed to high temperature, the protein denaturation occurs. |
| Avoid temperature fluctuation | Do not fluctuate the temperature of the pre-filled syringes and vials. If repeating warming and cooling occur, the antibodies may become instable, aggregate and leads to reduced shelf life. |
| Cold chain transportation | During shipping and distribution, the vials and pre-filled syringes filled with CGRP monoclonal antibodies must be kept in validated cold chain system and should maintain the temperature at 2°C to 8°C. |
Conclusion:
In a nutshell, it is concluded that the migraine management advances into 2025, CGRP monoclonal antibodies are one of the most powerful and scientifically proven treatment despite the other treatments. For the patients, you have different types of CGRP monoclonal antibodies are available. You can inject them at your home but if you want to inject them under the supervision of doctor, then you have also the option available. For the manufacturers, the manufacturing of CGRP monoclonal antibodies require the demand of sterile bioprocessing systems and regulatory compliance. AIPAK ENGINEERING is providing you world class solution and pharmaceutical grade machinery that is effective in the manufacturing of purest form of CGRP monoclonal antibodies. For more information, contact us!
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours

