Nutritional Injection Unit: The Complete FAQ Guide In 2025
Have you thought why nutritional preparations are getting popularity day by day? Because these preparations enter straight into the blood stream and reach the systemic circulation in larger amount. These preparations are available in vials, ampoules, BFS bottles etc. and made under aseptic conditions. Nutritional injection unit is accountable for providing these nutritional formulations to you and this proves how importance this unit holds itself.
In this guide, you get the understanding about introduction, kinds, benefits, applications, important considerations and challenges of nutritional injection unit. It is also necessary to discuss different nutritional preparations this unit holds and what packaging options are utilized by these preparations.
1.What do you understand by nutritional injection unit?
Nutritional Supplement Prepared By Nutritional Injection Unit-Picture Courtesy: Hodgson Pharmacy
Nutritional injection unit is specially designed equipment used to produce parenteral nutritional preparations in aseptic conditions. It has great importance in pharmaceutical industry and offers safe and effective manufacturing of nutrient-rich formulations. These nutrient preparations are crucial part of treatment of many individuals like unconscious patients, pregnant ladies, athletes, chronically-ill peoples, malnutrition children etc. Such nutritional supplements are given to fulfill nutrient deficiencies of these people.
2.What are the favors of nutritional injection unit?
Nutritional injection unit holds chief place in pharmaceutical industry and proves to be very advantageous for producing a quality product with great safety and efficacy.
Uniformity & precision:
Producing Nutritional Preparations With Great Uniformity & Precision-Picture Courtesy: Nuceria Health, In Miami, FL
Nutritional injection unit is capable of producing nutritional preparations with great uniformity and precision. Every step is performed properly to ensure a quality product. Automatic machines are utilized, that results in minimization of human errors in production.
Aseptic environment:

Restricted Access Barrier System (RABS) Used In Nutritional Injection Unit
Clean rooms, sterilize-in-place (SIP) system, clean-in-place (CIP) system and restricted access barrier system (RABS) are used to ensure aseptic conditions during preparation of nutritional formulations. This feature makes nutritional injection unit an ideal choice for making sterile preparations. Other factors like temperature, moisture, light and dust are kept controlled and a favorable atmosphere is provided for contamination-free manufacturing.
High productivity:
Nutritional Injection Unit Shows High Productivity
This nutritional injection unit contains automated equipment that gives you high productivity by rapid filling of nutritional preparation and reduces human interference.
Versatility:
Nutritional Injection Unit Deals With Vials, Ampoules, Prefilled Syringes, BFS Bottles-Picture Courtesy: Nipro Pharma Corporation
Nutritional injection unit provides you production of nutritional preparations contain in different packaging containers. This unit deals with vials, ampoules, pre filled syringes, BFS bottles and adaptable to various batch sizes and formulations.
Regulatory compliance:

Nutritional Injection Unit Follows Good Manufacturing Practice (GMP)-Picture Courtesy: Single Use Support
Nutritional injection unit is compliant with regulatory standards and follows good manufacturing process (GMP). This property ensures that the preparation of nutritional supplements is carried out in optimistic environment.
Low waste production:
Nutritional Injection Unit Supports Low Waste Production-Picture Courtesy: CORR- Healing And Recovery
Nutritional injection unit performs all process of manufacturing with great efficiency and this result in less material loss during production. In this way cost efficiency enhances with time.
3.What nutritional preparations are supported by nutritional injection unit?
Nutritional preparations are sterile formulations and packaged in vials, ampoules, BFS bottles etc. They demand aseptic conditions and efficient equipment for production. Nutritional injection unit has it all to fulfill the requirements of nutritional formulation manufacturing.
Vitamins:
Vitamins have vital importance for good health of body. Their deficiency can lead to serious health conditions and consumption of these vitamins can cure ailments. This is the reason nutritional injections of certain vitamins are available in market, to facilitate the administration of these necessary nutrients into the body and to acquire quick effects of them.
Vitamin supplements present as injectable are mentioned below:
| Vitamin supplements | Image | Role |
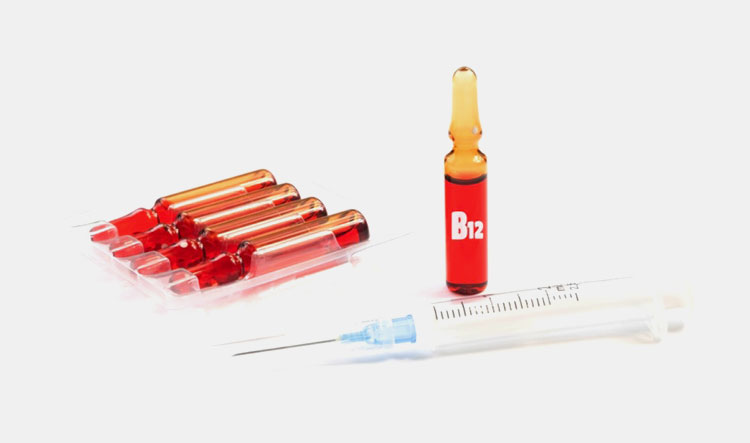
| Vitamin B12: | 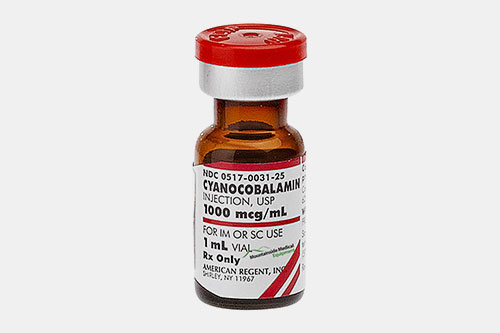 |
This vitamin is also known as cobalamin and helps in boosting energy. It also has role in improving health of nerves and blood cell. |
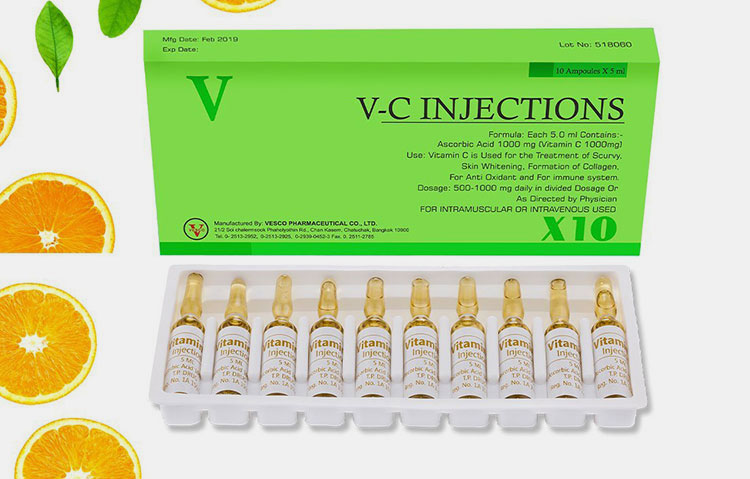
| Vitamin C: | 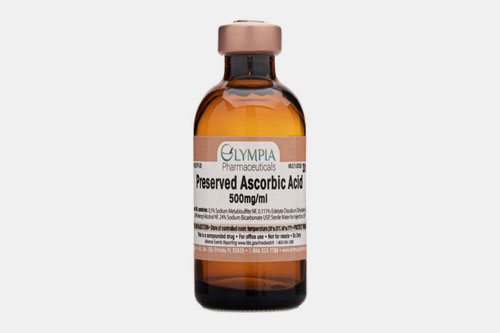 |
This vitamin is also known as ascorbic acid. It has predominant role as anti-oxidant. It enhances the immunity and collagen formation. |
| B-complex vitamins: | 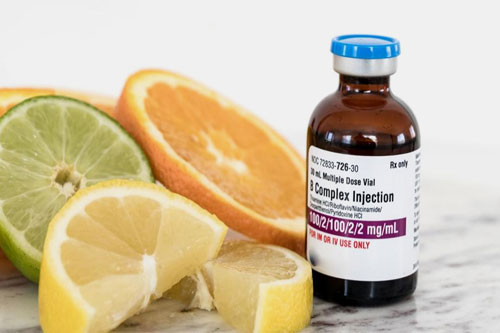 |
This complex comprises of vitamins including
B1: also known as thiamine, it establishes nerve and muscle function. B2: Another name for this vitamin is riboflavin. It has role in energy production. B3: it is also called as niacin. It improves circulation and metabolism of body. B5: it is known as pantothenic acid. It participates in hormone and cholesterol synthesis. B6: it is called as pyridoxine. It supports brain development and mood regulation. |
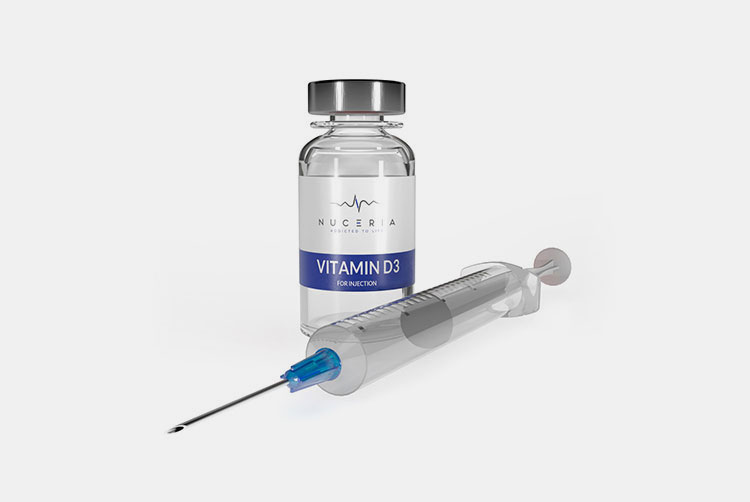
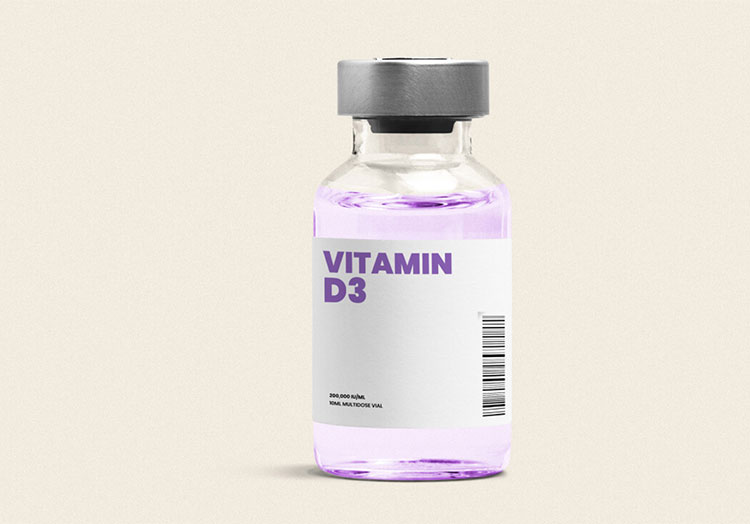
| Vitamin D: |  |
Vitamin D3 is available in injectable form. It has significant role in enhancing bone health and immunity. |
Minerals:
Minerals play significant role in maintaining various processes in body. Supplements of magnesium, iron, calcium etc. are available as nutritional formulations and nutritional injection unit helps to manufacture these nutrient rich preparations.
| Mineral supplements | Image | Role |
| Calcium: |  |
It has crucial role in maintaining bone strength and nerve signaling. |
| Magnesium: |  |
It improves muscle function, relieves stress and regulates heart rhythm. |
| Zinc: |  |
It enhances skin health and immune system. |
| Iron: |  |
It has chief role in transportation of oxygen, which contributes in further important processes of body like formation of hemoglobin etc. |
Amino acids also hold great importance and their suitable amount must be present in body for carrying out various functions. Available nutritional preparations of amino acids are:
| Amino acid supplements | Image | Role |
| Glutamine: | 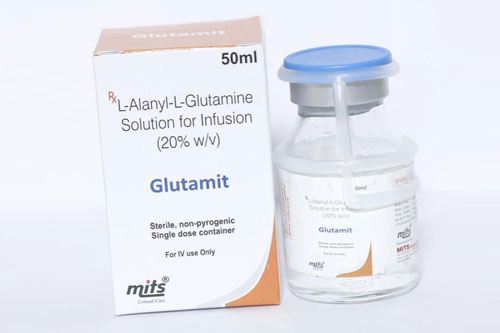 |
It improves gut health and contributes in muscle recovery. |
| Arginine: | 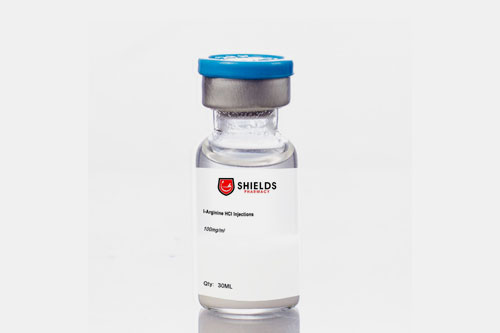 |
It supports cardiovascular health and enhances blood flow. |
| Taurine: |  |
It acts as antioxidant and enhances heart and brain function. |
| Carnitine: |  |
It has role in fat metabolism and energy production. |
| BCAAs (leucine, isoleucine, valine): | 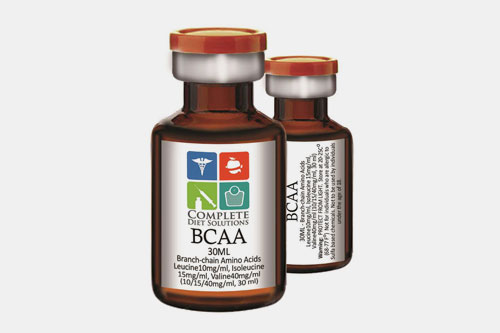 |
It has dominant effects on muscles. It supports muscle repair and endurance. |
Antioxidants:
Antioxidants are agents which protect cells from action of free radicals. Damage by free radicals can lead to dangerous disease like cancer. Antioxidants are available as nutritional preparations to overcome the need of body by administering them parenterally.
| Antioxidant supplements | Image | Role |
| Glutathione: | 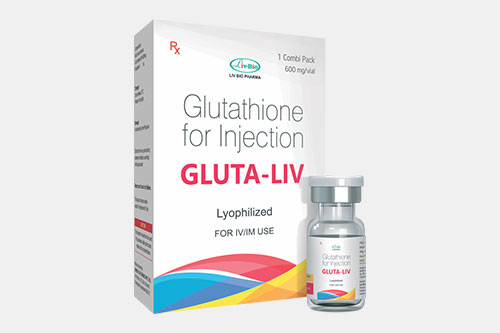 |
It has significant role as detoxifier and anti-aging. |
| Alpha-lipoic acid: |  |
It saves cell from destruction caused by oxidative stress. |
| Coenzyme Q10: |  |
It has dominant role in cellular energy production and maintaining heart health. |
4.What is the manufacturing process involved with nutritional injection unit?
Nutritional injection unit involves the manufacturing of nutritional preparations like vitamins, minerals, amino acids etc. Before starting the process, composition of formulation is defined means in how much concentration nutrient supplement is required. Make sure that the ingredients are compatible with each other to avoid any chemical reaction in future. Production of nutritional preparations follows below process:
Sourcing of raw materials:

Water For Injection USP-Picture Courtesy: B. Braun
Procure active ingredient like vitamins, amino acids etc. and carrier fluids such as water for injection/saline of pharmaceutical grade or USP standard. Water treatment system is used to purify water that is used in preparation of formulation. Inspect that the ingredients are suitable for sterile preparation or not, as they must be of high quality and contains no endotoxins.
Preparation of sterile solution:

Preparation Of Sterile Solution Is Carried Out In Clean Room
The process of making sterile solution in taken place in a clean room as temperature, moisture, etc. are kept controlled for providing aseptic conditions for production. Required active ingredients are dissolved in water for injection or saline by using mixing vessel. Benzyl alcohol is also added in multi-dose preparation as preservative.
Sterile filtration:

Sterile Filtration For Pharmaceutical Manufacturing-Picture Courtesy: Brother Filtration
Prepared solution is passed through sterile filter in order to remove the bacteria and unwanted particles from it. As you are preparing a nutritional preparation that directly goes in to the blood, so it is integral that you must use sterile and pyrogen-free equipment for filtration.
Filling:

Filling Of Vials-Picture Courtesy: Steriline
Aseptic filling line is used to fill the sterile solution into required container. Commonly used containers are ampoules, vials, BFS bottles and IV bags. Filling line is used according to packaging container and automated equipment is preferable for large production.
The process of filling starts with introduction of sterile solution into filling line. The packaging containers are moved towards filling line by conveyor, where precise amount of solution is filled into the container by the help of filling nozzles. Some nutritional formulations like glutathione are sensitive so they are lyophilized to keep them stable throughout the shelf life after filling them into the vial. Lyophilizer is incorporated with vial filling line to provide seamless production.
Sealing:

Ampoules Are Sealed By Flame-Sealing-Picture Courtesy: Marchesini Group
After filling, containers are sealed by appropriate techniques. Rubber stopper and aluminum seals are used to seal vials. Flame sealing is performed to seal ampoules. Heat sealing is done to seal IV bags.
Sterilization of final formulation:

Sterilization By Autoclave-Picture Courtesy: Shutterstock
Nutritional preparations that are heat resistant are sterilized by using autoclaves. This step is performed to prevent any chance of contamination. Other nutritional preparations that are heat sensitive are not sterilized by autoclaves protecting the formulation from getting degraded by heat.
Inspection:

Inspection Performed Manually-Picture Courtesy: Pharma’s Almanac
Inspection is carried out to detect the presence of particles, discoloration of formulation or leakage. Automated or manual both methods are used for inspection.
Labeling & packaging:
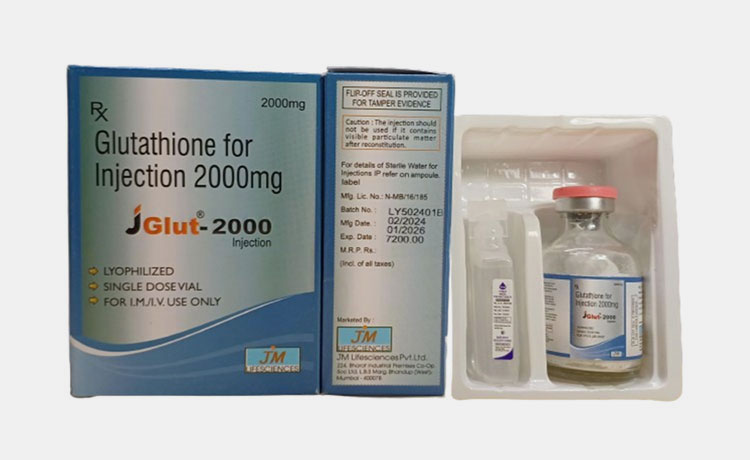
Labeled and packaged glutathione preparation
Proper labeling is essential in order to avoid any trouble at time of administration. Label must contain composition of nutrient, batch number, dates of production and expiration and storage conditions.
For secondary packaging, cartoning machines are used. Boxes are often used to packaged vials and ampoules. Aseptic conditions are maintained while packaging the final product.
Quality control & testing:

Sterility Testing-Picture Courtesy: Charles River Laborateries
Quality control is paramount for preparation of sterile formulations. Certain tests are performed to ensure that the formulation is up to the mark. The tests which are included are sterility test, endotoxin test, pH & osmolarity test, assay and stability testing.
Storage & distribution:

Refrigerator Is Used To Store The Nutritional Preparations At 2-8 C
Storage condition must be kept favorable for maintaining the stability of final product. Nutritional preparations are refrigerated at 2-8 C. heat-sensitive drugs are protected from light exposure completely. These preparations must be distributed under good distribution practices (GDP).
5.What are the packaging options of nutritional injection unit?
Nutritional preparations are produced by nutritional injection unit. The packaging options used in manufacturing of these preparations are good enough to handle sterile preparations. Vials, ampoules IV bags and BFS bottles are used as packaging containers.
Vials:
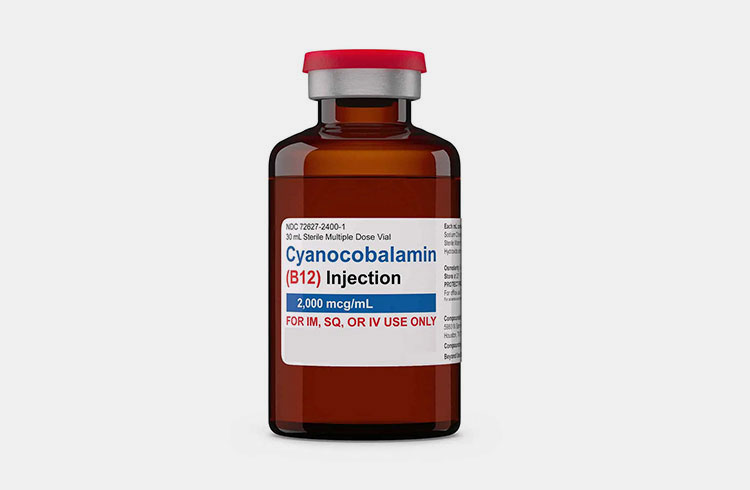
Vitamin B12 Preparation Dispensed In Vial-Picture Courtesy: Drp IV
Vials are denoted as small containers, made up of glass or plastic. These containers are used to dispense sterile liquid or freeze-drying powder of nutritional preparations. They are capable to keep formulation sterilized until they are administered. Vials can be single-dose or multiple-dose, depend on the need of formulation.
Formulation present in single dose vials must be utilized after opening due to absence of preservatives.
Preparations in multi-dose vials can be used within a certain time after un-sealing because benzyl alcohol is added as preservative in it.
Glass vials are commonly used for nutritional preparations because these are heat & chemical resistant.
Ampoules:
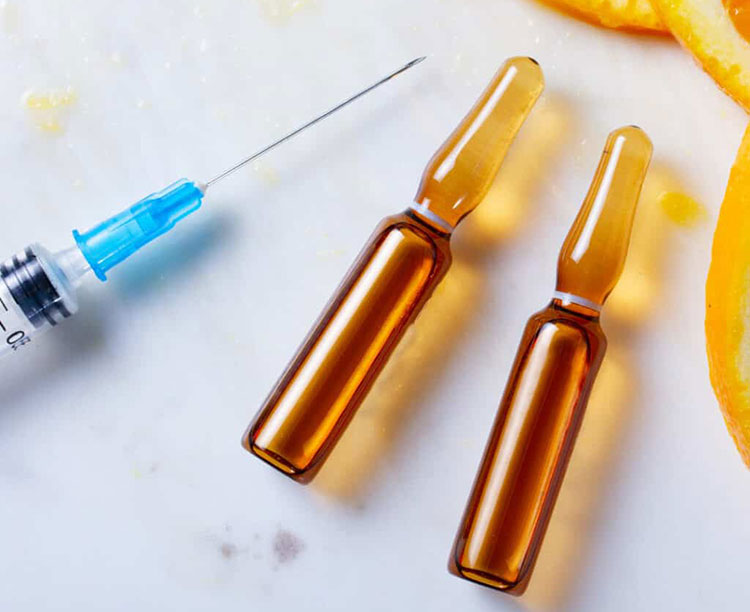
Vitamin C Containing Ampoules-Picture Courtesy: Vie Aesthetics
Ampoules are the small, sealed glass containers, intended to be used for once. Single doses of nutritional preparations like vitamins, minerals, antioxidants etc. are dispensed in ampoules.
These ampoules are made of clear or amber glass. These are opened by breaking the neck at a pre-scored line. These containers are capable to keep formulation sterile till the administration. It protects delicate nutrients like vitamin C and has minimal chances
Of contamination as it is single-use.
IV bags:
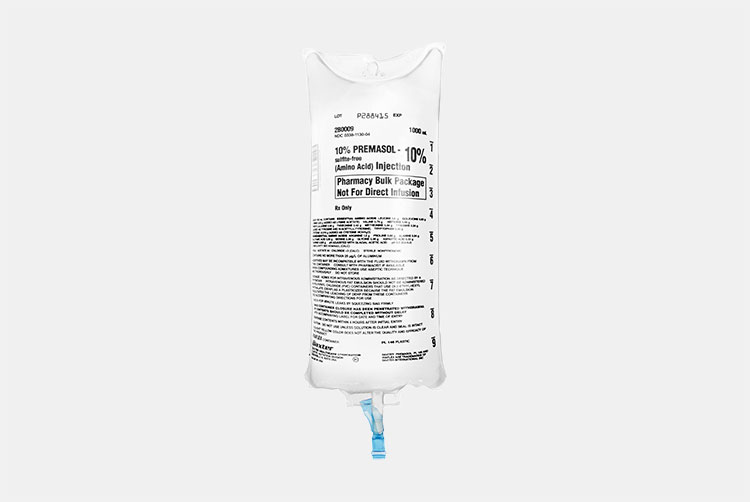
Amino Acid Preparation Packaged In IV Bag
Intravenous bags (IV) are flexible containers, made up of plastics like PVC or polyolefin. These containers are used to hold sterile nutritional preparations like amino acids. Other than nutritional preparations, dextrose 5%, normal saline, or water for injection are also dispensed in it. Nutrients such as iron, zinc, magnesium, vitamin C, glutathione, B-complex vitamin are mixed in these fluids for infusion.
BFS bottles:

BFS Bottle Containing Amino Acids-Picture Courtesy: B. Braun
Blow-fill-seal bottles (BFS) bottles are sterile, small plastic containers. These are produced by using special method called as blow-fill-seal technology. These containers are flexible and tamper-proofed. It is used in one time so no chance of contamination.
Ready to use amino acid or vitamin C preparations are packaged in this container. Moreover, nutrient support fluids are also dispensed in it.
6.What key machines are involved in production process carried by nutritional injection unit?
Production of nutritional preparation demands for efficient machines, as target is to produce a safe and potent formulation. Machines that are used in all over process of manufacturing are well-equipped to create a top quality product.
Water treatment system:

Aipak water treatment system
This water treatment system is used to manufacturer water for injection. It is made up of stainless steel and delivers water of high purity. It is an appropriate choice for treating water which is used in nutritional preparations. This system is easy to maintain and consumes low energy, so proves to be cost-effective too.
Mixing vessel:

Mixing Vessel
Mixing vessel is used in pharmaceutical industry to form sterile solution of nutritional formulation. This mixing tank is made up of stainless steel to avoid any reaction between the vessel and the components of formulation. It contains magnetic stirrers to facilitate the homogenous mixing of ingredients in to water for injection. It is very crucial to maintain aseptic conditions while carrying out the mixing of components.
Vial filling line:

Aipak Vial Filling Machine For Liquids
Nutritional preparations are dispensed in vials, so for this step vial filling line is used. This equipment consists of four important parts including vial washing station, sterilizing station, filling and stoppering station and capping station. Some nutritional preparations are sensitive so they are lyophilized before dispensing.
For freeze-drying powders, another vial filling machine is used which has incorporated lyophilizer after filling and stoppering station and vials are first filled & stoppered then freeze-dried by the help of lyophilizer. These vial filling machines shows high efficiency and follows GMP production standards.

Aipak Vial Filling Machine For Lyophilized Powders
Ampoule filling line:

Aipak Ampoule Filling Line
Nutritional preparations are filled in ampoules for single-dose preparations. This filling line comprises of bottle washing machine, sterilizer and ampoule filling and sealing machine. It can handle ampoules of size 1-20ml and all steps are performed by automated machines. It shows high productivity and meets GMP standards.
BFS bottle filling line:

Aipak BFS Bottle Filling Line
Nutritional preparations like amino acids, vitamin C, etc. are delivered in BFS bottles. For filling of these nutritional preparations, bottle BFS production line is used. This production line works on blow-fill-seal technology, which means in single continuous process, bottle is formed then filled with sterile solution and then sealed. All the process is carried out under aseptic conditions.
IV bags filling line:

IV Bags Filling Line-Picture Courtesy: Comecer
Nutritional preparations and carrier fluid are dispensed in IV bags. For filling these formulations in IV bags, above filling line is used. It consists of four important parts that includes decontamination station, where pre-sterilized bags are decontaminated from outside. After that feeding station is present, in which IV bags are made ready to move to filling station. In filling station, IV bags are filled with preparation and get sealed in two-step process.
Autoclaves:

Autoclave Used In Pharma Industry-Picture Courtesy: HVAX
Autoclave is basically a vessel like equipment that utilizes high pressure saturated steam at temperature 121 C – 134 C to sterilize the products. It holds important place in sterile formulations production. Nutritional preparations are sterilized after filling and sealing to avoid any possible contamination.
Labeling machine:

Aipak Labeling Machine
Labeling machine is used to apply printed labels on containers of nutritional preparations. Label must contain information like product name, composition, volume, route of administration, batch number, expiry date etc.
Cartoning machine:
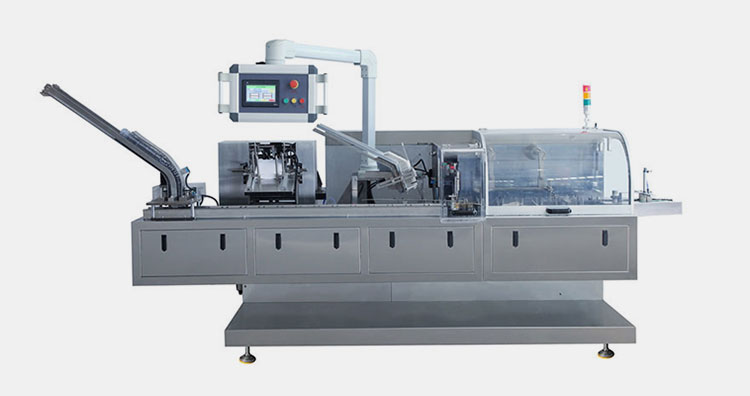
Aipak Cartoning Machine
Cartoning machine is used for secondary packaging of nutritional preparations. The finished product is packed in boxes to keep it safe during distribution and storage.
7.Can you elaborate about important considerations of nutritional injection unit?
Important Considerations Of Nutritional Injection Unit-Picture Courtesy: Sisu’amor
To understand nutritional injection unit thoroughly, it is necessary to know about the important considerations of this unit.
| Important considerations: | why you need to consider: |
| Aseptic conditions & sterility: | Maintaining aseptic conditions is paramount for nutritional injection unit. All process must operate in a clean room. This room contains HEPA filtration which filters air and don’t allow the bacteria, dust etc. to reach the room.
Keep sterility intact is another important thing. Sterile filtration and SIP and CIP systems are used for this purpose. |
| Accuracy: | Accurate dosing of nutrients in nutritional preparations is integral. Every nutrient must be present in suitable amount for safety of patient. High efficient dosing pumps and automated mixing system is required to get accuracy in procedure. |
| Compatible with different containers: | Nutritional preparations are dispensed in different containers. So equipment must be compatible with these packaging containers. |
| Regulatory compliance: | All process of production must meet the standards set by regulatory bodies. In this way it becomes easier to maintain the efficiency and productivity of operation which results in high quality product. |
| Stability of product: | In order to keep product stable, initiatives must be taken. Sensitive nutrient products need cautious handling like nitrogen flushing is done to prevent oxidation, contamination and other unwanted reactions. Other than this UV protection is also necessary. |
| Operator safety: | Nutritional injection unit must be design in a way that it requires less human contact. Safety interlocks or contamination alarms must be present to avoid any risk of contamination in product. |
| Easy to clean & maintain: | Nutritional injection unit must be easy to clean so that cleaning of inner and outer parts become un-interruptive. Secondly, it must be got dissemble easily making maintenance convenient for manufacturer. Spare parts should be available to avoid hindrance in operation. |
8.What challenges can be faced by using nutritional injection unit?

What Can Be The Challenges Of Nutritional Injection Unit?-Picture Courtesy: Depositphotos
Like any other production unit, nutritional injection unit also contains some challenges that need to be resolved on time to avoid delay in production. This topic possesses information about challenges and their reason of happening along with appropriate solution to give you awareness about such issues.
| Challenges | Reason | Solution |
| Inaccurate filling: | Fault in flow meters or pump.
Misaligned or deteriorated of filling nozzles. |
Calibrate pumps, flow meters or filling nozzles.
Replace worn-out filling nozzles. |
| Detection of contamination: | Fault in SIP /CIP systems.
Substandard cleanroom discipline. |
Validation of cleaning cycle must be carried out.
Monitoring of cleanroom is carried out to ensure standard aseptic conditions. |
| Presence of precipitates in final product: | Inappropriate mixing.
Incompatible pH or interaction between ingredients. |
Adapt mixing protocols according to good manufacturing process (GMP) and calibrate mixing process.
Look for change in mixing order to avoid precipitation. |
| Leakage in packaging container: | Poor sealing process.
Low grade packaging material. |
Calibrate the sealing process according to mandatory standards.
Procure packaging material from certified suppliers and inspect the container before using in operation. |
| Blockage in filters/pipelines: | Increase particulate load or clumping of material.
Improper cleaning of inner parts between batches. |
Pre-filter the raw material and exceed frequency of filter change.
Deep cleaning and validation of whole system must be carried out. |
| Machine stops working: | Poor functioning of sensor or PLC.
Power fluctuation. |
Replace sensors or inspect control system for errors.
Alternate source like UPS system must be there to compensate power failure. |
| Label is missing or place incorrectly: | Labeling machine misalignment. | Calibrate labeling machine. |
Conclusion:
Nutritional injection unit is integral part of pharmaceutical industry which provides you a sterile, effective and potent product to fulfill nutrient deficiencies of your body. Chief considerations of this unit are accurate formulation, aseptic conditions, automated equipment and compliance with regulatory standards. When these aspects are focused, high productivity is achieved. Still there are some challenges encountered during operation of this unit, which can be overcome by quick maintenance. Are you desirable for getting more authentic knowledge? AIPAK Engineering provides genuine information regarding nutritional injection unit, you can contact us anytime!
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours

