Varicella Vaccine Production: The Complete FAQ Guide In 2025
Are you wondering what the varicella vaccine is made of? Do not worry- You will be equipped with all this knowledge. Varicella vaccine is made from varicella-zoster virus. This virus is grown in human cells safely and effectively. In the early period, there was no cure for chicken pox, and a lot of people died of it. It has been produced for curing chicken pox, recently.
The tiny and strong worrier is made after the research of years. A virus that causes fever, small blisters and itchy skin is now weakened and killed by a vaccine enclosed in vials. There are various steps in varicella vaccine production and each step I perfect which you can see in its effectiveness.
This has cured millions of people and taken them out of misery. If you are looking for vaccine to cater chicken pox then this FAQ guide will provide all the information that needs to be known. So, yeah let’s uncover it.
1.What is the varicella vaccine?
Varicella vaccine-Picture courtesy: childhealthy.com
Varicella vaccine is made from varicella-zoster virus that is actually weakened in the lab to be transferred it in a strong tool that destroys or kills its companion- a living varicella-zoster virus. This tiny virus is actually behind chicken pox. Varicella vaccine consists of weakened varicella-zoster virus(attenuated) which produces antibodies in body against this virus and gives an immune system an arm to use when the real virus shows its presence.
2.What is the purpose of varicella vaccine production?
Vaccines are made on the basis of some of the purposes. The same goes for varicella vaccine production. There are various purposes for varicella vaccine production which are:
To treat chicken pox
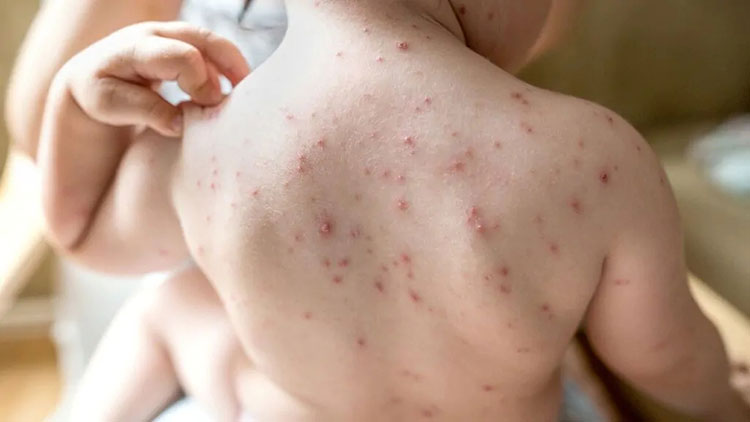
Chicken pox-Picture courtesy: clevelandclinic.com
Varicella-zoster virus causes an infection called chicken pox. You have to be cautious as this infection is transferred from person to person. Symptoms of chickenpox such as blisters, itchy skin and fever are treated by the varicella vaccine.
Declined death rates
When chicken pox was at high peak spreading, a lot of people lost their live. This mild infection turned into severe diseases such as nerve cell infection, pneumonia, and other bacterial infections and started causing death. This vaccine was introduced to combat such infections and reduce the death rates.
Prevent shingles risk

Shingle- Picture courtesy: myhealth1st.com
Shingle is a viral infection caused by a varicella-zoster virus. It is painful rash and appear anywhere on the body. This virus remains in body in inactive form after having chicken pox. There are chances of its activation in later years and cause shingles. However, the varicella vaccine can reduce risk of shingles developing later in life.
Strong immunity
Strong immunity
When a person takes varicella vaccine, the body develops strong immunity against the virus by producing antibodies for the causing virus. When a virus enters the body, antibodies immediately recognize and respond to the virus.
3.Describe the virus used in varicella vaccine production?
Varicella-zoster virus-Picture Courtesy: infectiondiseaseadvisor.com
A virus such as varicella-zoster is used in the production of varicella vaccine. It is used in an attenuated and weakened form, which is passed through various processes to make its strengthening powerless without its complete deactivation. Attenuated varicella virus stimulates the immune system and helps the body in the production of antibodies.
Does this virus cause infection in our body? No, you do not need to worry- it does not cause infection in your body. That is not how it works. In the laboratory, the enemy (harmful virus) is changed into a helper, active varicella virus to weakened varicella vaccine.
Varicella virus is a DNA virus and belongs to the family of Herpesviridae. This vaccine helps the body to fight back and neutralize the live varicella-zoster virus. It destroys the real enemy by helping body immune’s system.
4.When and how varicella vaccine production is made first time?

History of varicella vaccine
When
In 1970, chicken pox had wrapped Japan with its symptoms of blisters and itching all over the body of children. Among the children, there was a boy who also had gotten chicken pox, and her father’s name was Takahashi. He could not resist himself and started working on the treatment of chicken pox.
The first scientist who developed the varicella vaccine, in 1970, to treat the varicella zoster virus was a Japanese virologist named, Dr Michiaki Takahashi.
How
Dr Takahashi was at University of Osaka. Here, he started research on this. He isolated virus from the fluid blister of three-year-old boy, Oka. The virus strain is named Oka strain after this. The Oka strain was cultured in human embryonic lung cells and guinea pigs. It was tested on a small number of people but it still showed symptoms of chickenpox in these people. As it lost a bit of its strength.
What happened next? What he and his colleague did was use of attenuated method. They attenuated and weakened the virus, leaving with les strength but enough to stimulate the immune system to fight back varicella virus.
After a deep research and testing, in 1986 Japan declared its use for children of age between 10 to 12. Therefore, it became worldwide vaccine.
5.Describe the composition of varicella vaccine production?
Followings are the ingredients used in varicella vaccine production.
Attenuated varicella-zoster vaccine
It contains the dead version of varicella-zoster virus. It is weakened in nature but can equip immune system. When it is injected into the body, it is recognized as a malign foreign agent and starts producing antibodies.
Salts

Potassium chloride-Picture courtesy: tridentenergyintl.com
PH level is important for the stabilization of vaccine. Salts are used in the varicella vaccine to maintain the PH of the vaccine. Sodium chloride, potassium chloride and sodium sulphate are used as salt and buffer agents to maintain their chemical stability during storage.
Culture medium
It must be in your information that virus needs a medium to grow. A medium must be of living. DNA and protein are taken from the human diploid cell. These are used in varicella vaccine production because they are safe and reliable.
Neomycin

Neomycin
Neomycin is used in varicella vaccine production. To save the vaccine from germs, a sterile environment is needed. Neomycin is used to kill bacteria. Thus, it ensures that bacteria do not grow and pose problems in future.
Stabilizers
Sorbitol, a sugar, is used as a stabilizer. It is used to protect protein from damaging when temperature exceeds its optimal temperature. This is also used when freeze-drying at lyophilization process. Moreover, sucrose is used in varicella vaccine production to sure its effectiveness and activity.
6.What is the making process of varicella vaccine production?
Followings are the steps involve in varicella vaccine production. Let’s dive in it.
Selection of the viral strain
Varicella zoster virus strain
The first important step is the selection of viral strain. This strain is of varicella zoster virus. The tiny strain is behind the development of a varicella-vaccine to cure chicken pox.
It is passed through various process to make it attenuated. The reason behind strain weakening is that to makes it incapable of causing infection, but can replicate for vaccine production.
Preparation of cell culture
The virus is living so it needs medium to grow. Human diploid cells are used as a host cell and as a culture. These are loaded in bioreactor where a safe and optimal temperature is sustained for the growth of cells.
Production
Production
Once the cell culture is prepared, an attenuated virus is added to the solution. Here, the virus started infecting the host cell. With the passage of time and days, it replicates in numbers of viral particles.
Then, the needed outcome is collected and purified by using centrifuge to remove the extra materials such as host protein, DNA and dead cells. Varicella virus needs low temperature of 2 to 8 centigrade. So, it keeps itself stable, otherwise, at high temperature, it will be ineffective and break down.
Formulation
Formulation
The next important step is to add substances into the purified viral particles. These substances can be buffer agents, solvents and stabilizers. These help in maintaining the integrity and stability of vaccine while stored.
Packaging

Packaging
Sometimes, a vaccine is undergone for lyophilization to make it in dry-powder form. It is the last stage in the making process. Here, vials are washed and sterile. They are filled with formulated vaccines and sealed tightly to ensure no germs entry. Expiry date, usage instruction, ingredients information and other required information are labeled on vials. Finally, it is ready for cartooning.
7.Do you know about the machines used in varicella vaccine production?
In varicella vaccine production, high-quality machines are used to ensure safe and effective vaccine production. Are you curious to know? Let’s explore together.
Bioreactor

Bioreactor
Bioreactor is a large tank that provides all environmental conditions such as controlled temperature, PH, and other conditions suitable for its optimal growth. Bioreactors contain mixer to mix the solution, sensor to control every desired condition, main chamber and ports to load cell culture.
In the bioreactor, a human cell is loaded and then, a strain of attenuated varicella zoster virus is used. Virus starts invading the cells and making copies of themselves. By providing the sterile environment, ensures inactivation of the varicella virus. Without cell culture, you cannot prepare the varicella vaccine.
Bioreactor holds large volume of batch. So, you can get high yield at a short time.
Centrifuge machine

Centrifuge machine-Picture courtesy: mke-lab.com
Centrifuge machine holds an important place in varicella vaccine production. Centrifuge machine separates the debris and waste materials from the formed solution in a bioreactor.
Now, a question will pop in your mind that how centrifuge machine separates material? It uses a centrifugal force to separate a low- and high-density materials in centrifuge machine.
It consists of chamber in which the centrifugal process is happen, a motor to give energy to rotor and control panel to monitor temperature, speed and time. The sample is spin at high speed and heavier particles move at bottom while light attenuated virus stays upright in centrifuge.
Production line

AIPAK high ultrasonic vial production line
The above picture of varicella vaccine production line consists of major machines such as ultrasonic cleaning machine, sterilizing dryer, and filling machine. You can use the machines separately and in line as feasible to you.
You will know all the machines use in above production line. Let’s get started.
Ultrasonic washing machine
This machine is used to wash vials by using ultrasonic wave. It ensures a germs-free vials cleaning for effective storing of vaccines. Tank is filled with solution and vials that is to be clean. Then, a high frequency waves are shed to kill, if any, germs and microbes.
Sterilizing dryer
This machine is a dry heat sterilizer that is used to sterilize the vials using hot air and dry heat. Sterilization process is done to ensure no contamination and microorganisms left in vials. After sterilization, it is ready for filling with vaccine.
Filling machine
The important step is filling vials from vaccine. All this is done by using a filling machine. By using filling nozzle, each vial is accurately and precisely filled. Sensors are also used which monitor vials volume.
Pharmaceutical autoclave
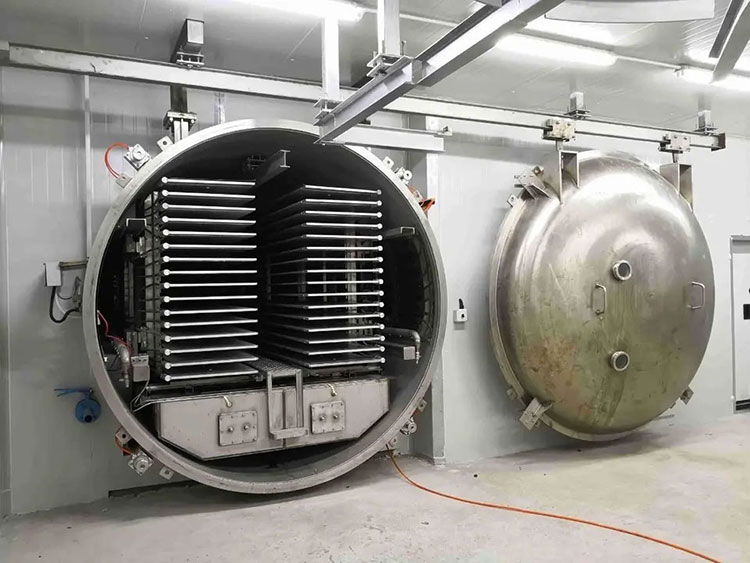
AIPAK pharmaceutical autoclave
This type of machine is also used in production of varicella vaccine. This is beneficial due to which you can store vaccine in a dry powder form for a longer time. you do not need to be worry about its degradation as at lyophilization, it is made a temperature stable.
By using freeze chamber, a solution is freeze at very low temperature. Vacuum system apply vacuum directly which results in sublimation process to change ice directly in vapors. Finally, a remaining moisture is removed from vaccine by gentle heating.
After drying, vials are sealed or stopper.
Labeling machine

AIPAK high speed and automatic labeling machine
At the end, labeling machine is used to label the vaccine vials. Labeling is important therefore one can know about the varicella vaccine. Batch number, ingredients usage, date before use and other information’s are labeled on vials.
8.Can you explain the therapeutic uses of varicella vaccine production?
Everything that has been invented comes with uses. Similarly, production of varicella vaccine is made to benefit you with the following therapeutic uses.
Treat chickenpox
Treat chickenpox
Chickenpox is caused by the contagious virus known as varicella zoster virus. This is severe in people who are not immune with vaccine, varicella vaccine. But people who are vaccinated with its vaccine are less likely to develop chickenpox. So, it treats chickenpox.
Prevent herpes zoster
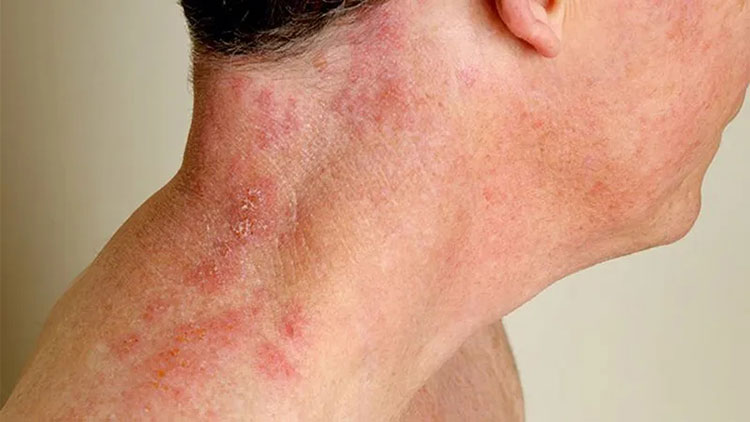
Herpes zoster
Herpes zoster (shingles) is caused by another virus but it can be prevented by varicella vaccine. It can control the shingle infection in person in early life.
Prevent congenital varicella syndrome
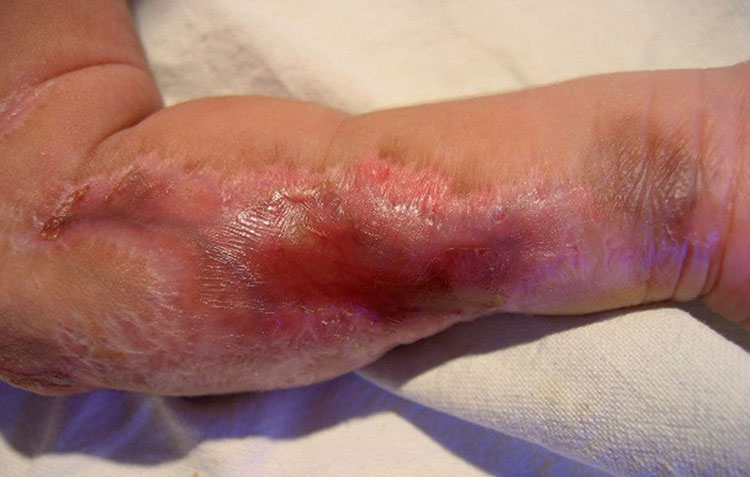
Congenital varicella syndrome
Any infection to a pregnant woman has an impact on the baby in womb. However, if
pregnant women are having chickenpox, there might be birth defects in baby called congenital varicella syndrome. Therefore, vaccinating women before pregnancy can prevent this syndrome by immune the body.
Encephalitis
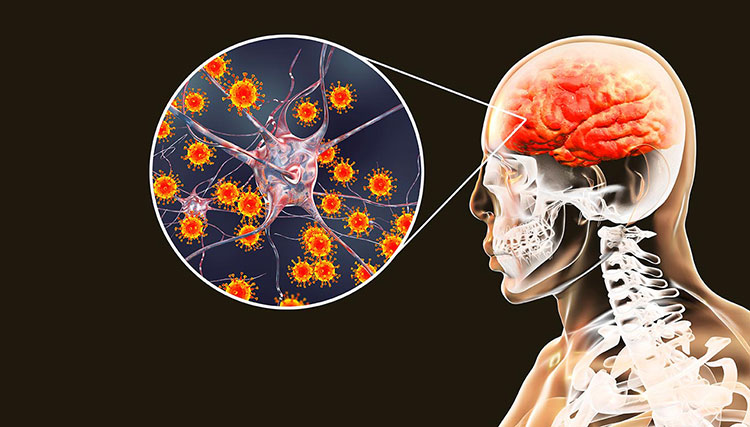
Encephalitis- Picture courtesy- gavi.thevaccinealliance.com
It is the brain inflammation caused by viral parasite. This disease is rare but serious. Varicella vaccine can reduce its symptoms such as headache, vomiting and confusion.
Varicella pneumonia

Varicella vaccine
When viral infection is not treated timely can spread in body. Similarly, when varicella virus spreads too lungs and results in their damage. Injecting a varicella vaccine can reduce its risk.
9.What are the side effects of overuse of varicella vaccine production?
Often, varicella vaccine does not have as such side effects when use in high amount but sometimes, overuse also cause side effects. What are you waiting for? Let’s get started.
Pain and redness
Pain, redness and rashes on the injected place can e seen. These are of mild level and go away on its own. Sometimes, the infected place gets swollen due to overuse of varicella vaccine.
Allergy
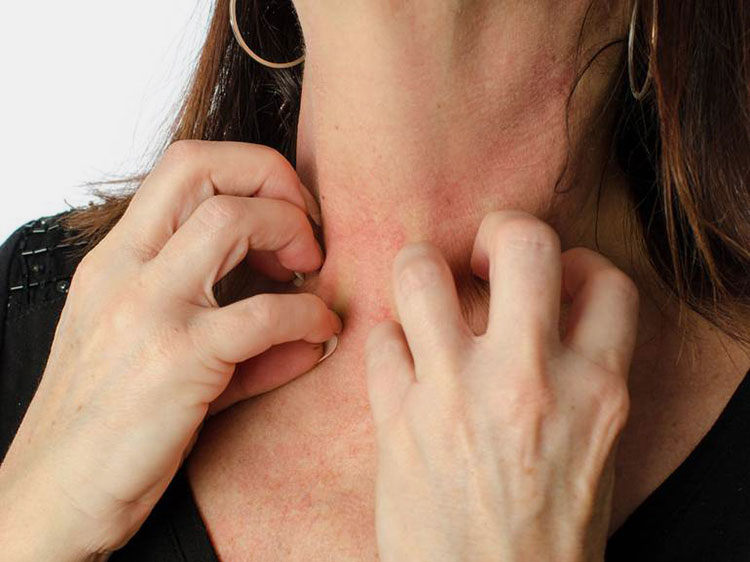
Anaphylaxis allergy- Picture Courtesy: healthline.com
People with sensitive and weak immune system can catch allergic reaction, this allergy can be a life threatening. Anaphylaxis allergic reaction is shown after overdose of vaccine.
Fever
Now you will think how fever is related with overuse of varicella vaccine? So, when you use vaccine over and over again, it put stress on immune system rather than providing benefits. It increases white blood cell production that trigger the body results in fever.
Risk of activation
Attenuated varicella virus is in dormant form in the body and can be, rare cases, activated. HIV and cancer patients are likely on the brink of virus activation.
Thrombocytopenia
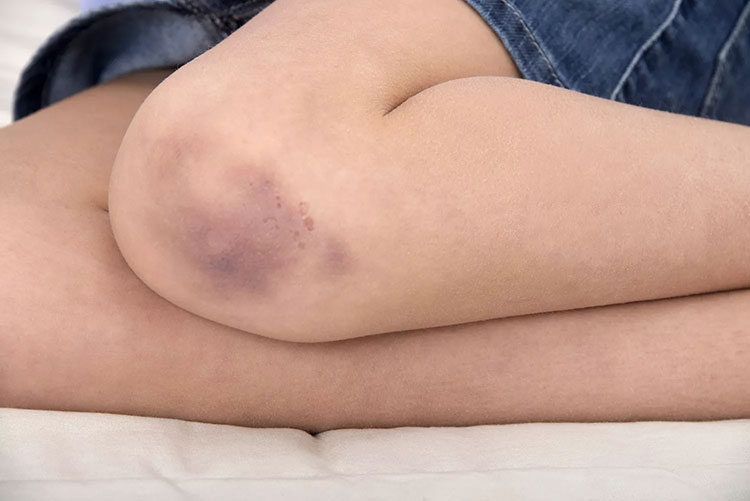
Thrombocytopenia
Thrombocytopenia is condition in which there is less platelets in the body. This case is verry rare. However, when a person injected this vaccine, its immune system misrecognizes its own platelets and start destroying them. The repeated dosing may trigger the condition (bruising and any kind of bleeding).
10.What are the challenges faced during varicella vaccine production?
As the production of varicella vaccine is very complex process. Therefore, it is bound with some of the challenges. Here you go.
| Challenges | ||
| Safety and efficacy
Safety and efficacy |
Varicella virus is passed through different process to make it weaken that it does not have the ability to infect cells. But, if it is too weaken then it would not be capable for use in vaccine and it, it is not get attenuated properly, it would e risk of its activation. | |
| Temperature sensitivity
Temperature sensitivity-picture courtesy: Thermo fisher |
Varicella vaccine needs low temperature to maintain itself. The temperature must be low otherwise a vaccine will lose its structure and will become ineffective.so, temperature maintenance is very difficult as sometimes it drops and waste vaccine. | |
| Vulnerability of contamination
Vulnerability of contamination |
Virus is being replicated in human cells. These cells require a perfect controlled environment for growth. Any contamination and bacteria can lead to wastage or the entire batch. | |
| Less global access
Less global access |
The cell culture is expensive. The vaccine itself is a bit expensive. Therefore, it is not widely use and results in low vaccination rate. | |
| Economic barriers | As you know that human diploid cels are used as a host cell for varicella vaccine production. These are very expensive and its availability is rare. Every batch produce little of viral solution. So, cost exceeds yield. | |
11.What are the quality control measures for varicella vaccine production?
Followings are the measures which should e taken that are necessary for a safe and purified production of varicella vaccine.
Cell line testing

Cell line testing-Picture courtesy: DNAforensics.com
You must come to know, as discussed above in the guide, that human cells are used for viral strain production. It must be checked that whether a human taken cell is germs-free or not and must contain stable chromosomes, to ensure a sterile environment for virus relipcation.
Raw materials testing
The very first thing that mut be checked is the raw materials sterility because on basis of usage of raw materials, a vaccine is produced. Stabilizers, buffer agents, solvents and growth media are tested. To check invisible microorganisms, mycoplasma detection is done.
Testing of viral seed
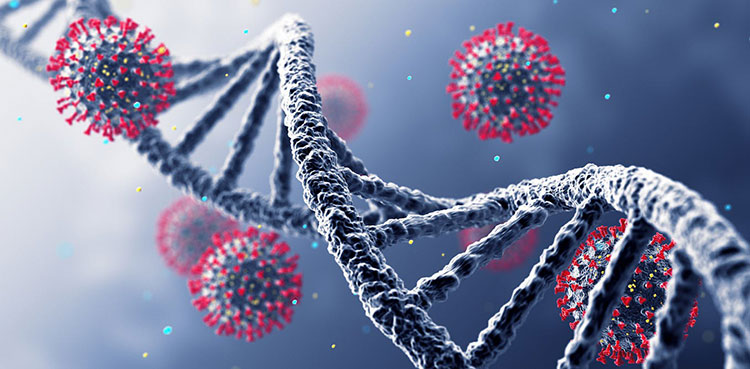
DNA sequence-Picture courtesy: aconveration.com
A viral strain such as attenuated varicella virus is used to process all the production. This viral seed is tested to ensure no foreign pathogen or microbe is attached to it or not. DNA sequence is also monitored. The whole process depends on it. Any little contamination can lead a whole batch wastage.
Final product testing
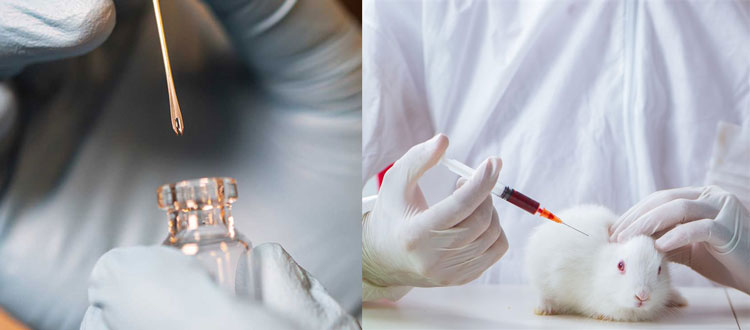
Final product testing
When a varicella vaccine is produced, it undergoes various tests.
- A PCR test is done to make sure that virus contains the exact virus strain.
- A sterility test is done to ensure no pathogen contamination.
- A biochemical analysis is done to check any protein and DNA debris and protein.
- A final product is tested on the animals to check negative reactions.
Comply international standards

Comply international standards
It must be ensured that the vaccine production must comply with international standards. Vaccine must be submitted for the final review to the WHO, FDA, WOAH etc. for goal quality assurance.
Conclusion
To conclude the guide, it is to be admitted that science and technology have been proved fruitful and save humanity on the brink of problems. A varicella vaccine has helped millions of people from chickenpox by immunizing the immune system against varicella zoster virus. This vaccine requires low temperature to remain stable and maintain its effectiveness. If you have any queries, you are always welcome on the AIPAK Engineering website.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours