Rabies Vaccine Production: The Complete FAQ Guide In 2025
Are you aware of the consequences you could face after getting caught by the rabies virus? If yes then you surely value the rabies virus production. Rabies is a fatal disease that can affect your nervous system very badly and has some initial symptoms like encephalitis, hydrophobia and fever which later advances to seizures, hallucinations and paralysis. This dangerous disease spreads through bite or scratch by infected animal like dogs to humans or other animals. Rabies vaccine is given to avoid the prevalence of rabies infection.
This guide helps you to get knowledge that is necessary for rabies vaccine production including the components of rabies vaccine and the steps involving it. This topic also sheds light on the noteworthiness of rabies vaccine production and also tells you about future prospects of it.
1.What is meant by rabies vaccine production?
Rabies Vaccine Production-Picture Courtesy: Gavi, The Vaccine Alliance
Rabies vaccine production, as the name indicates, is the process of making vaccine to combat with rabies virus. Rabies disease is caused by rabies virus, which is a neurotropic disease that leads to hydrophobia, fever, seizures, hallucination, paralysis and at last death. Rabies vaccine provides immunity against it; hence rabies vaccine production secures an important place as it is saving many lives from this deadly disease.
2.How beneficial is rabies vaccine production for the society?
Rabies vaccine production is crucial for good health of society. It benefits the humans as well as animals in so many ways. This topic is designed to provide some knowledge upon advantages of rabies vaccine production to highlight its importance.
Prevention from a deleterious virus:
Rabies Is A Life-Threatening Virus-Picture Courtesy: Business Standard
Rabies disease causes thousands of death every year worldwide, last year reported fatalities reach to fifty nine thousand. Such huge numbers of deaths defines the worth of rabies production as it offers protection from such life-threatening virus.
Impact on healthcare system:

Administration of vaccine to infected animals-picture courtesy: Bryan county news
Rabies vaccine production has noticeable impact on healthcare system. Administration of rabies vaccine to infected animals like dogs will be helpful in decreasing the cases of rabies disease.
Pre exposure and post exposure safety:
Rabies Vaccine Administration-Picture Courtesy: Crest Pharmacy
Rabies vaccine production is integral especially in countries that have vast number of rabies cases. This vaccine provides pre-exposure protection; means it is given to people that are at high risk of meeting this harmful virus like veterinarians, animal handlers, travelers to rabies spreading place or laboratory technicians. Also it gives post-exposure protection in cases when individual is bitten by infected animal.
Animal health safety:

Rabies vaccine administration to livestock-picture courtesy: farm progress
Animals like pets or livestock gets administered with rabies vaccine to avoid the happening of rabies disease. In this way rabies vaccine production protects animals against serious illness.
Economical privileges:
Rabies Vaccine Production Is Favorable To Economy-Picture Courtesy: Express Tribune
Reduction in rabies disease cases by rabies vaccine production has profound effects on economy. This initiative saves money that spent on post-exposure prophylaxis treatment. It also diminishes the losses in livestock productivity.
Growth in biotechnology:

New Technologies Utilization In Rabies Vaccine Production Contributes To Growth In Biotechnology-Picture Courtesy: Southern California University Of Health Sciences
Recently new technologies are utilizing in rabies vaccine production, followed by making innovations in cell culture, virus inactivation and recombinant technologies. Such initiative also contributes in development of other vaccines.
3.What is the history of rabies vaccine production?

First Rabies Vaccine Was Made By Louis Pasteur & Emile Roex-Picture Courtesy: Britannica
After getting knowledge about the favorable role of rabies vaccine production, you are surely thinking about when this valuable thing was made for the first time? Well history of rabies vaccine production is inspiring quest and this topic provides you an insight in it.
In 1885, for the first time rabies vaccine production had taken place. Louis Pasteur and Emile Roux created rabies vaccine by the help of spinal cords of infected rabbits. This vaccine contained myelin, a nerve protein as it comprised of nerve tissues. So many side effects were reported after utilizing that vaccine.
During 1960-1970, cell-culture based rabies vaccine production was occurred, that marked as breakthrough in history of rabies vaccine production. It has less side effects and more effective against rabies disease.
4.What types of rabies vaccine are made by rabies vaccine production?
Basically, rabies vaccine production is carried out by two methods, first technique is rabies vaccine production by nerve tissues which is less effective and has many side effects so not in practice. While other technique of rabies vaccine production is carried out by using cell culture and it is recommended due to less side effects and more efficacy.
Rabies vaccine production by cell culture method involves the growth of virus in specific cells under controlled environment of laboratory, which proceeds by inactivation or attenuation, purification and then formulation in to a vaccine. It has three types stated below:
Purified vero cell rabies vaccine:
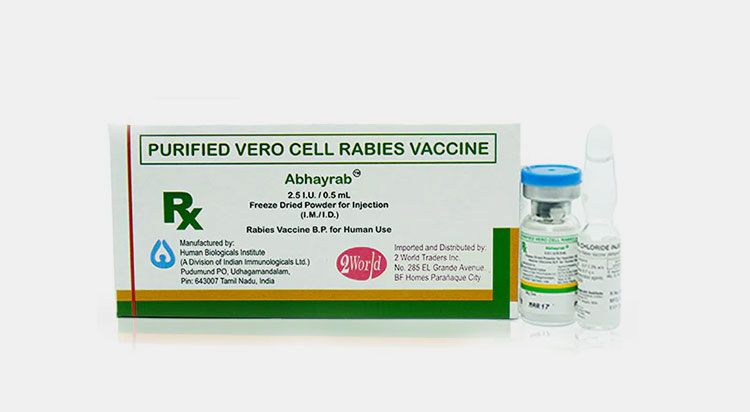
Purified Vero Cell Rabies Vaccine
It is considered as safe and highly efficient rabies vaccine. It is developed by growing virus in vero cells taken from monkey kidney cells, after that virus is inactivated and vaccine is purified. It is commonly used before and after exposure prophylaxis treatment in humans.
This vaccine is available in lyophilized powder, which is reconstituted with sterile water. It is given intramuscularly and stored at 2-8 C. For pre-exposure prophylaxis treatment, three doses are given on day 0, day 7 and day 28, while post-exposure prophylaxis treatment is given for five days on day 0, day 3, day 7, day 14 and day 28.
Purified chick embryo cell rabies vaccine:
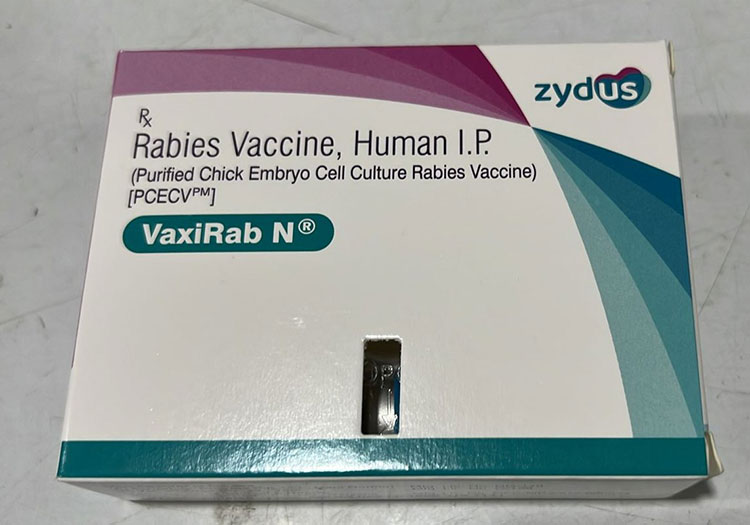
Purified Chick Embryo Cell Rabies Vaccine
Purified chick embryo vaccine is commonly used for pre-exposure and post-exposure prophylaxis treatment. It is developed by growing virus in chick embryo cell, then virus is inactivated and vaccine is made purified. It is made in lyophilized powder and constitutes again by using sterile water and stored at 2-8 C. It is given in 2 or 3 doses for pre-exposure prophylaxis and 5 doses for post-exposure prophylaxis treatment.
Human diploid cell rabies vaccine:
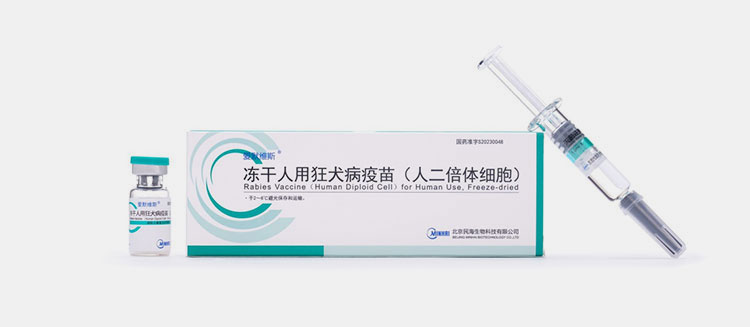
Human Diploid Cell Rabies Vaccine
This vaccine is made by growing virus in human diploid cells taken from human fetal lung tissues, after that virus in made inactivated and vaccine is purified accurately. It is administered intramuscularly and given in 2-3 doses for pre-exposure prophylaxis and 5 days for post-exposure prophylaxis. It is provided in lyophilized powder and store at 2-8 C. It is considered as safe and is highly recommended.
5.What components are utilized in rabies vaccine production?
Rabies vaccine production holds an important role in constructing a healthy population. Now the question arises what things constitute this vaccine? This topic tells you about main components that are utilized in rabies vaccine production.
| Components | Information |
| Strain of rabies virus:
Picture Courtesy: News-Medical.Net |
A weakened or inactivated rabies virus strain is required. Virus is grown in controlled environment of lab and then inactivated by using inactivation agent. |
| Cell culture:
Picture Courtesy: Nikon Healthcare |
Different cell cultures are used to grow virus in different types of vaccine. Human diploid cells are extracted from human fetal lung cells.
Chick embryo cells are taken from chick embryo cells. Vero cells are drawn from monkey kidney cells. |
| Inactivation agent:
|
This agent is used to inactivate the virus while keeping its immunogenicity intact.
For this purpose beta- propiolactone is used. |
| Material for purification:
Centrifuge |
Filters, centrifuges etc. are used for purification of vaccine from cell debris and virus particles. |
| Stabilizers:
|
Stabilizers are added in vaccine to keep it stable during shelf life and ensure its potency.
Examples are sorbitol, human albumin, gelatin |
| Preservatives:
Picture Courtesy: Scientific Laboratory |
Preservatives are added to make vaccine stable and prevent contamination caused by bacteria or fungi.
Commonly it is added in multi-dose vaccine. Example includes thimerosal. |
| Adjuvants:
|
Adjuvants are incorporated in rabies vaccine to increase its response to vaccine antigen.
Example includes aluminum hydroxide. |
| Sterile diluent:
|
Sterile diluent is required to reconstitute the vaccine which is present in dried powder.
Commonly used sterile diluent is water for injection. |
6.What steps are involved in rabies virus production?
It becomes very necessary to understand the steps that are involved in rabies virus production in order to make its production more efficient. Steps of rabies vaccine production are defined below:
Selection and cultivation of virus:
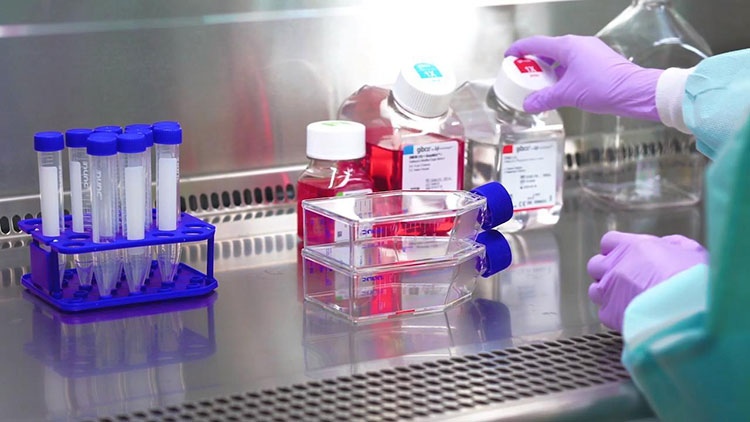
Virus Cultivation-Picture Courtesy: Thermos Fischer Scientific
An appropriate strain of virus is selected to perform rabies vaccine production. Virus seed is prepared under controlled conditions. Suitable cell-culture system like human diploid cell, chicken embryo cell or vero cell is prepared in large bioreactors or flasks to grow virus in it. Virus seed is cultivated and conditions are kept favorable for growth of virus.
Collection of virus:
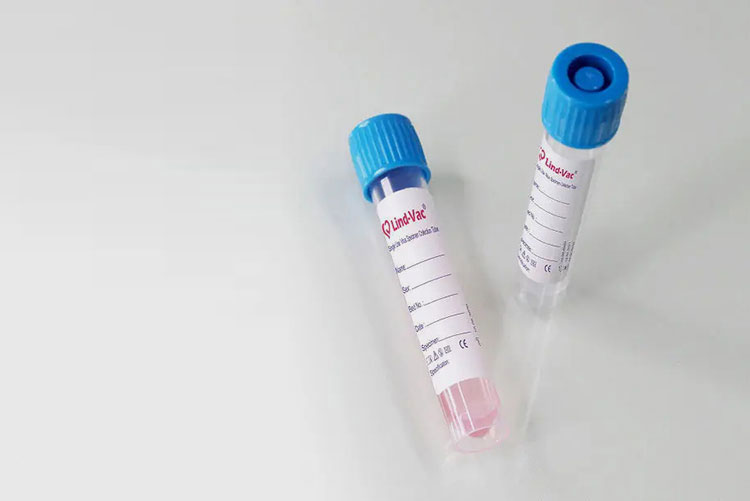
Viral Specimen Collection-Picture Courtesy: IntraVec Technology
After allowing many days for growth of virus, virus-rich fluid is collected and commonly cells are cleaved to release virus present in it.
Inactivation of virus:
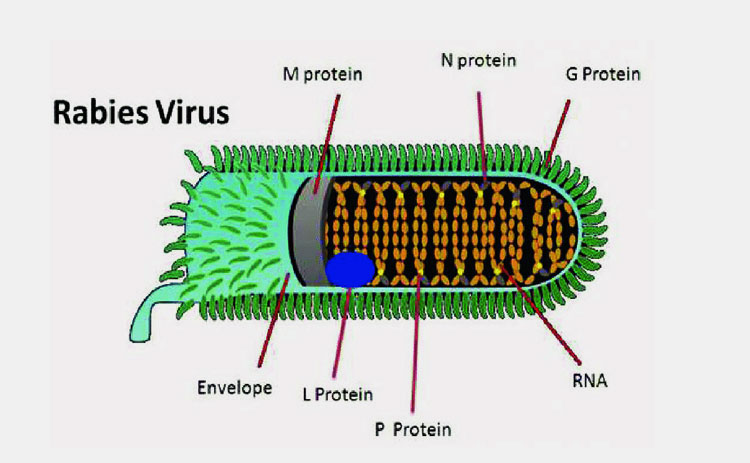
Rabies Virus Is Inactivated To Prevent Its Harmful Effects While Maintaining Immunogenicity-Picture Courtesy: ResearchGate
Virus is inactivated by utilizing inactivation agents in rabies vaccine production. This step is integral to secure the immunogenicity of virus while making it harmless. Commonly used inactivation agent is beta propiolactone.
Purification of vaccine:

Purification Of Virus-Picture Courtesy: CP Lab Safety
Vaccine is purified from impurities to ensure its safety and potency. Techniques involved to purify the vaccine are ultra-filtration, centrifugation and chromatography. These techniques have pivotal role in keeping vaccine stable and effective for pre-exposure and post-exposure treatment.
Addition of stabilizer and adjuvant:

Sorbitol Powder Is Used As Stabilizer
Stabilizer and adjuvant are added in vaccine formulation to keep it stable and immunogenic. Vaccine is prepared in lyophilized form to avoid contamination. Sometimes preservatives are included in multi-dose formulation to avoid instability during shelf life.
Filling & packaging of vaccine:

Rabies Vaccine Is Filled And Packaged In Vials And Pre-Filled Syringes
Dried powder of vaccine is filled in vials or pre-filled syringes. Sterile diluent most probably water of injection is available along with vaccine to use it for reconstitution of rabies vaccine.
7.What are the machines used in rabies vaccine production?
Machines are the integral part of any production and to understand any process, we also need to know about the machines. This topic contains knowledge about important machines used in rabies vaccine production.
Bioreactor:

Bioreactor-Picture Courtesy: Home-Biotop
Bioreactor is the crucial part of large scale rabies vaccine production. and ensures a high quality and stable rabies vaccine. Its role is discussed below:
Role in preparation of cell culture:

Cell Culture Preparation In Bioreactor
On large scale, bioreactors are utilized to grown host cells for the growth of virus like vero cells, human diploid cells, and chick embryo cells. Without the proper preparation of cell culture you cannot continue rabies vaccine production. Controlled environment was maintained for suitable cell culture preparation.
Role in viral growth:

Viral Strain Is Introduced In Bioreactors
When host cells are prepared, viral strain is introduced in to bioreactor. Virus starts growing under controlled environment.
Bioreactor characteristics:

Characteristics Of Bioreactor-Picture Courtesy: European Biotechnology
Controlled environment means conditions are optimum for cell culture preparation and viral growth. Temperature, pH, oxygen, nutrient level and agitation, all the things are favorable to facilitate the process.
Bioreactors have closed system so chances of contamination are very low and it offers you stable and safe vaccine production of top quality.
Bioreactors are capable to hold large batches, so it becomes convenient to make huge amount of viral antigen.
Lyophilizer:

Lyophilizer Used In Pharma Industry-Picture Courtesy: Syntegon
Lyophilizer is machine used to perform freeze drying and it is essential for rabies vaccine production as vaccine is formulated in dry powder (lyophilized) form. This form helps to remain stable during shelf life. Lyophilizer removes water content from vaccine by the help of a process called lyophilization (freeze drying).
Lyophilizer is incorporated in vial filling line.
Important components of lyophilizer:

Lyophilizer Has A Chamber To Hold Vials-Picture Courtesy: Berkshire Sterile Manufacturing
Lyophilizer has a chamber to hold the vials or trays during the process of freeze drying.
Temperature controlled shelves are present to freeze and heat the product.
Condenser is used to trap water vapor by freezing it.
Vacuum pump has function to reduce the pressure in order to facilitate sublimation.
Control system is present to control temperature, pressure and time. It is automated to keep the process efficient.
How it works?

Vaccine Is Converted In To Freeze Dried Powder By Lyophilizer-Picture Courtesy: Manufacturing Chemist
Vaccine is filled in vials and subjected to freezing at low temperature i.e. -40 or lower. Vacuum is applied and heat is incorporated to sublimate the ice. Remaining moisture is removed by further heating.
Vial filling line:

Aipak Vial Filling Line
Vial filling line is used to fill and package rabies vaccine in vials with great precision. It can be semi-automatic or fully automatic. It ensures
Aseptic conditions during whole process which is utmost requirement of sterile products like rabies vaccine.
How it works:
Rabies Vaccine Filled In Vials-Picture Courtesy: Healthy Pets
First of all vials are washed from inside and outside with sterile diluent like water for injection at vial washing station.
Vials are then sterilized to remove endotoxins by heating them at high temperature, commonly 250 C.
Vaccine is filled in sterilized vial with great precision and rubber stopper is partially inserted upon vials before subjecting them to lyophilizer.
Lyophilizer performs freeze drying of vaccine present in vials to keep it stable during shelf life.
Lyophilized form of vaccine filled in vials is then moved to capping station, where vial get sealed with aluminum caps. After capping, inspection is carried out through manual or automated inspection machines. This step is necessary to detect the problem in filling or sealing of vial.
Sealed vials after inspection move to labelling and packaging station, where labels are applied that contains important information need to be known. Like ingredients, batch number, strength, expiration. After labeling, vials are packaged into cartons or trays.
Pre-filled syringe filling line:

Pre-Filled Syringe Filling Line
Pre-filled syringe filling line offers aseptic and highly efficient way to fill and seal sterile products like vaccine. It has certain benefits like convenient to use, dose accuracy and low contamination chances.
How it works:
A Pre-Filled Syringe-Picture Courtesy: IBSA
Firstly, syringes are sterilized by heating them at high temperature and then place them in tub/nest. Tub and its lid are decontaminated under laminar flow in tub decontamination unit.
Sterilized syringes are moved to filling and stoppering station to fill the vaccine which is lyophilized by the lyophilizer before into syringes and then a plunger stopper is inserted under vacuum conditions to reduce air bubbles.
A backstop clip is also incorporated to prevent plunger being pulled out.
Pre-filled syringes are then moved to labeling station and printed labels are applied on them that contain information like bar code, batch number, expiration etc.
Inspection is done by visual or automated machines to ensure accurate filling and sealing of formulation.
Pre-filled syringes are placed back in tub or nests to get packaged in blister packaging or trays for further packaging.
8.What are the critical aspects of rabies virus production?
Rabies vaccine production, a very significant process for the maintaining the healthy population but it has some aspects that are needed to be monitored with time as they have key role in the process. This topic is all about stating critical aspects of rabies vaccine production.
Selection of viral strain:
Rabies Virus Strain-Picture Courtesy: News-Medical.Net
Viral strain selection id first and most important step and it need to be done with care. Viral strain must be well-described and safe to give accurate results. Strain must possess good immunogenicity and poor pathogenicity.
Choosing cell culture:
Choose An Appropriate Cell Culture-Picture Courtesy: Wikipedia
Choosing a suitable cell culture is integral for accurate rabies vaccine production. There are three available cell cultures as you have read it before, vero cells, chick embryo cells and human diploid cells. Choose cell culture which favors your vaccine production the most.
Aseptic conditions:
Aseptic Conditions Are Required For Rabies Vaccine Production
Do you have any idea how sterilization is important in rabies vaccine production? This vaccine will be running in blood of a human so it needs to be safest. Aseptic environment must be maintained during whole rabies vaccine production and inspect environment about any contamination chances regularly.
Controlled environment:

Controlled Environment-Picture Courtesy: Allied Cleanroons
Controlled environment supports good cell culture preparation and viral growth. Optimum temperature, pH, oxygen and nutrients level contribute in highly efficient product.
Virus inactivation:
Rabies Virus
Virus inactivation must be done accurately to remain safe from deleterious effects of virus. Meanwhile its ability to generate an immune response must remain intact.
Purification of vaccine:

Purification Of Vaccine By Centrifugation-Picture Courtesy: Akadeum Life Science
Purification of vaccine from cell debris, cell proteins and impurities is necessary to maintain the sterility of product. Ultrafiltration, centrifugation, or chromatography must be carried out to purify vaccine.
Stabilization of rabies vaccine:
Stabilizers Are Added To Make Stable Formulation
Stabilization of rabies vaccine must be made sure by adding stabilizers. It is utmost requirement of formulation to keep it stable during shelf life.
Batch testing:

Batch Testing For Safe And Potent Product
Batch testing must be carried out to gain uniform quality and safety. Potency tests, sterility tests, safety tests etc. must be performed to ensure that the product meets mandatory standards.
Regulatory compliance:

Must follow guidelines told by WHO
Rabies vaccine production must follow good manufacturing practices to achieve an effective and high quality product. Guidelines must be followed that are given by WHO and national regulatory authorities.
Safety of workers:

Workers Must Be Vaccinated As Pre-Exposure Prophylaxis-Picture Courtesy: European Biotechnology
Individuals that are involved in rabies vaccine production must be vaccinated as pre-exposure prophylaxis and must know proper handling of components.
Storage:

No Cold Chain, No Immunization-Picture Courtesy: UNICEF
Storage conditions must be optimistic to maintain the product potency. Rabies vaccine is stored at 2-8 C, so cold chain storage units are necessity of rabies vaccine production.
9.What advance technologies can be utilized in rabies virus production?
Researchers are making efforts all around the world to bring advancements in recent procedures and to make them more efficient. Similarly, some advance technologies are in practice to make rabies vaccine production more consistent and established. This topic will give you information about advance technologies that can be utilized in rabies vaccine production.
Recombinant DNA technology:
Recombinant DNA Technology
Rabies vaccine production is made safer and efficient by using recombinant DNA technology. For your knowledge, Rabies virus glycoprotein, which is also called G-protein, is the main component that works as antigen and induces immune response. This G-protein is taken out from rabies virus and inserted into a small circular DNA molecule called plasmid vector. This plasmid vector is then placed into a host cell that can be yeast, insect or mammalian cell.
Host cell utilizes this DNA molecule to form massive number of rabies G-protein, which after proper purification, uses as antigen in rabies vaccine production. Adjuvants are also added to make immune response of vaccine stronger, in this way you get non-infectious, safe and potent rabies vaccine.
Viral vector technology:
Viral Vector Technology
This method is practiced in rabies vaccine production by benefiting with genetic engineering. Basically, a genetically modified virus which is not the actual virus is used to transport rabies virus genes into host cell, establishing the immune response without causing infection.
Transdermal delivery of rabies vaccine:
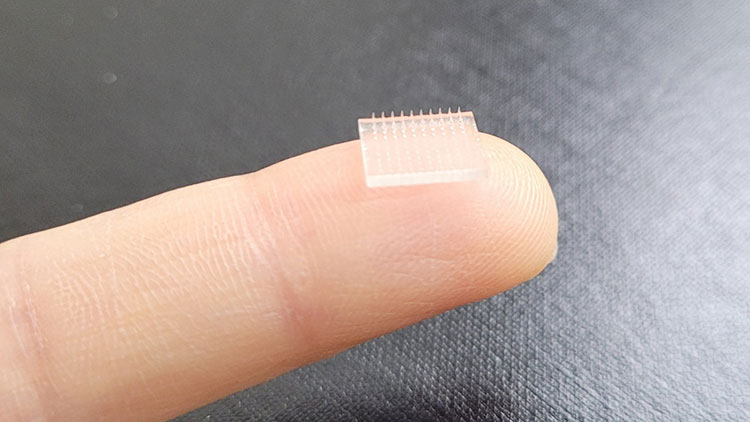
Microneedle Patch Use For Transdermal Treatment
Rabies vaccine is administered intramuscularly in to the body but a breakthrough is achieved by delivering the rabies vaccine through skin. Iontophoresis is coupled with microneedles to deliver the vaccine trans dermally. It offers painless procedure which is safe and effective too.
10.What challenges are faced during rabies virus production?
Rabies vaccine production is so advantageous for society. Many modern methods are utilizing to make it more efficient but there are still some challenges that need to be sort out. This topic provides you awareness about such challenges to draw your attention towards it.
Need for regulatory compliance:

Regulatory Compliance-Picture Courtesy: Tripwire
Rabies vaccine production demands for complete regulatory compliance and approval is necessary from regulatory bodies to continue the process. This thing limits the production and distribution of rabies vaccine.
Cold chain requirement:

Cold Chain For Rabies Vaccine Storage
Rabies vaccine production requires a cold chain to store the rabies vaccine at 2-8C, this requirement makes things complicated as maintaining the storage conditions in remote or low socio-economic places is quite difficult
Use of animals in research:

Use Of Animals In Research-Picture Courtesy: QPS
Ethical considerations about use of animals for the testing of rabies vaccine production become a hurdle to continue it smoothly.
11.What are future prospects of rabies virus production?
This topic talks about some interesting future prospects that can be made to enhance the productivity and efficiency of rabies vaccine production.
mRNA vaccine technology:
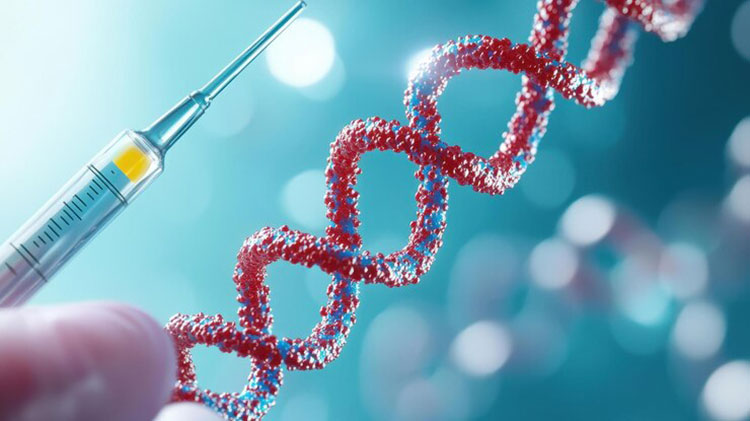
mRNA vaccine Technology
This technology involves the use of messenger RNA (mRNA) to command the cells to form a viral protein that induces the immune response. Researchers know the gene coding of glycoprotein present in rabies virus, which provokes immune response. Messenger RNA (mRNA) is synthesized in lab and glycoprotein gene is transcribed in it. This mRNA is encapsulated in lipid nanoparticles to keep it safe and facilitates it to enter human cells.
When mRNA enters cells, production of rabies virus glycoprotein starts. Body considers it as foreign and induces the defense system. Production of antibodies takes place that protect the person from rabies virus in future.
Use of artificial intelligence in rabies vaccine production:

Incorporation Of AI In Rabies Vaccine Production-Picture Courtesy: Itchronicles
Artificial intelligence (AI) is being used in every field to make things easier and efficient. In rabies vaccine production, AI is used to create algorithms that are helpful to predict viral mutations and supports re-making of vaccine according to variations. This contributes in more effective rabies vaccine production with great safety profile.
12.What storage conditions are used in rabies vaccine production?
Storage conditions have significant role in keeping product stable and potent. These conditions must be maintained to acquire high quality product. There are some factors that need to be monitored in storage conditions.
Temperature control:

Temperature Must Be Maintained Up To 2-8 C-Picture Courtesy: Freepik
Temperature must be maintained during storage of rabies vaccine production up to 2-8 C and final product also store at same temperature. Cold chains are required to store rabies vaccine but freezing must be avoided as it deforms the vaccine.
Protection from light:
Ultraviolet (UV) Light-Picture Courtesy: Regency Insight Blog
Light can cause degradation of rabies vaccine so exposure to light especially UV light must be avoided.
Regulatory compliance:

Good Manufacturing Practice Must Be Followed During Rabies Vaccine Production-Picture Courtesy: Arena Solutions
Rabies vaccine production must follow guidelines provided by WHO and national regulatory authorities like FDA for good manufacturing practice (GMP) and storage system.
Shelf life:
Rabies Vaccine Is Formulated In Lyophilized Form-Picture Courtesy: Avin Darou
A step involves in rabies vaccine production in which stability tests are performed under different conditions to determine the shelf life of rabies vaccine. Shelf life of rabies vaccine is estimated to be 2-3 years if proper refrigeration is maintained throughout storage. Rabies vaccine is formed in lyophilized form to keep it stable and sustain slight variation in conditions. As rabies vaccine reconstitutes, it should be used immediately or in few hours as per guidelines.
Conclusion:
Rabies vaccine production has a lot of benefits for society but it demands controlled environment for achieving the high quality product that must be safe, effective and stable. Important steps include cultivation of virus, inactivation of virus, purification, and formulation. Strict following of good manufacturing practice is made necessary to attain stable and potent product. By utilizing advance technologies in rabies vaccine production, safer and more efficient vaccines are producing. But still needs more development to tackle with challenges that are faced during production. For more information related to machine or topic, you can contact our team.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours