WFI System:The Complete FAQ Guide In 2025
Are you the newcomers to the market for WFI system? Then you may know that the purified water is important for pharmaceutical process. The quality of the water for injection can never be compromised. Pure water and water for injection in the pharmaceutical industry require strict production processes and environments.
Without a good understanding of water for injection, production problems can easily go wrong. This complete FAQ guide focuses on WFI and WFI system and answers questions about its importance. Keep reading and get your answers now!
1.What Is WFI And WFI System?
WFI-sourced: techsafety
WFI is water for injection. It is a kind of high-purity water specially used in the pharmaceutical and medical industries. This kind of water needs to meet strict production standards and quality without any impurities and chemical pollution. WFI can be used for various human injections, other animal injections, medical device cleaning, various parenteral, ophthalmic and other uses.
WFI system-sourced: crbgroup
The WFI system is a system specially used for preparing water for injection. It includes different types and parts. They can produce a variety of high-purity sterile water for injection drugs, sterile liquids and other purposes that meet strict standards. The various cleaning, sterilization, distillation and other processes are adopted.
2.What Kind Of Water Can WFI System Produce?
What Kind Of Water Can WFI System Produce-sourced: hameln-pharma
The WFI system mainly produces high-purity sterile water. Various antimicrobial agents are added to reduce contamination after opening. High-purity sterile water usually contains 0.9% benzyl alcohol. This is done to dilute active pharmaceutical ingredients that are administered intravenously, intramuscularly or subcutaneously.
3.What Are The Main Applications Of WFI System?
There are three main applications of WFI system, and they are:
Pharmaceutical industry
Pharmaceutical industry-sourced: customvalveconcepts
In the pharmaceutical industry, the most widely used application of WFI system is water for injection. Unlike ordinary drinking water, water for injection in the pharmaceutical industry does not contain any impurities and minerals. Because it is used in the human body through intravenous, intramuscular or subcutaneous injection, the quality requirements are very high.
Healthcare industry
Healthcare industry-sourced: mountainside-medical
In addition to the highest quality pure water required for infusion, in the healthcare industry, various wound flushing, medical device flushing, eye cleaning and the production of health care liquid products require the highest purity sterile water.
Skin care products industry

Skin care products industry-sourced: trambellir
The main ingredient of many skin care products you often use is sterile water. From cosmetics to your body care products, shampoo, shower gel, lotion, essence, etc., all use high-quality sterile water as the main solvent and ingredient.
4.What Are The Standard Criteria For Qualifying Water As WFI?
The production process, technology and subsequent quality inspection of water for injection have relevant standards:
Europe

Europe-sourced: edqm
Europe has relatively strict standards for water for injection. It only requires you to use a distillation process to prepare high-purity sterile water.
United States and Japan

United States and Japan-sourced: tsoshop
The United States and Japan have relatively loose requirements for water for injection. In addition to using a distillation process, they also allow the use of membranes and other purification processes.
Other requirements are similar:
- The conductivity should be less than 1.3 mS/cm;
- The total organic carbon content of organic compounds in the water system is not allowed to exceed 500 ppb;
- Endotoxins that can cause fever in patients should be less than 0.25 EU/mL;
- The bacterial or total aerobic microbial count should be less than 10 CFU/100mL;
- The pH value should be between 5.0 and 7.0;
5.What Are The Ways To Obtain WFI?
You can obtain water for injection by the following two methods.
Hot distillation (hot WFI)
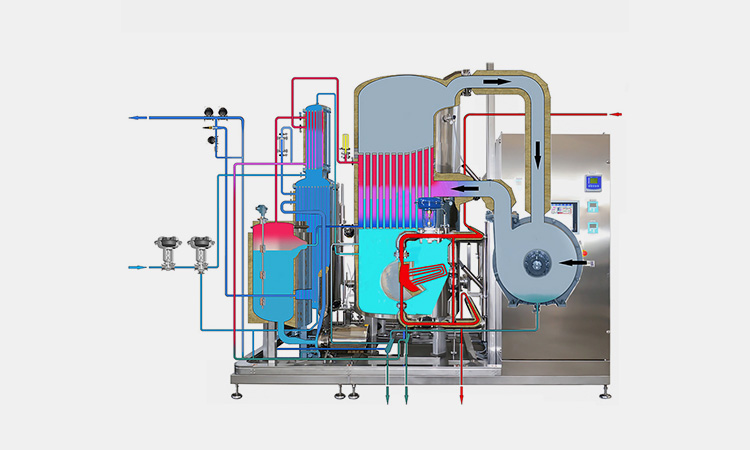
Hot distillation (hot WFI)-sourced: puretech
Hot distillation is a vapor compression distillation method, which is also known as hot WFI. It is the most supported method in Europe. This method basically uses the method of heating water, evaporating it and finally condensing it to obtain high-purity sterile water. Hot distilled water has the best quality and is completed under high temperature conditions throughout the process, meeting all high quality standards.
Reverse osmosis, electrodeionization and ultrafiltration methods (cold WFI)
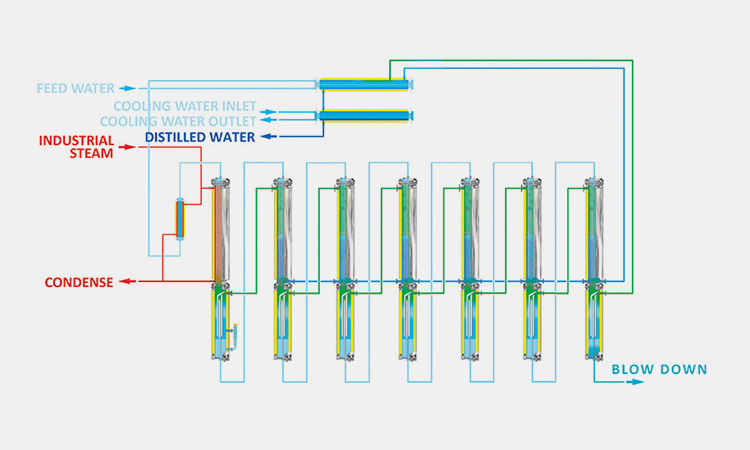
Reverse osmosis, electrodeionization and ultrafiltration methods (cold WFI)-sourced: puretech
Reverse osmosis, electrodeionization and ultrafiltration methods are relatively popular methods in the United States recently. It is also called membrane technology. The main process steps of this method are reverse osmosis, electrodeionization and ultrafiltration. Its main components are filters and membranes.
6.What Are The Two Main System To Produce WFI?
There are two main systems to produce water for injection.
Distillation system (hot system)

Distillation system (hot system)-sourced: puretech
Distillation system consists of a distiller. A distiller may consist of 2 to 8 distillation columns. The efficiency of distillation is mainly determined by the efficiency and number of distillation columns. During the heating process, water vapor is evaporated and then condensed to remove impurities and pollutants in the water.
Membrane system (cold system)

Membrane system (cold system)-sourced: puretech
Membrane system mainly refers to the use of filters and membranes to remove most of the unwanted molecules and particles without using heating. Then, electricity is used to remove positive and negative ions in the water. Finally, ultra-fine membranes are used to filter out pollutants.
7.What Are The Main Parts Of WFI System?
Main parts of distillation WFI system (hot system):

Main parts of distillation WFI system (hot system)-sourced: pharmaceutical-technologies
①Heat exchanger
It consists of multiple heating steel pipes. When it is preheated, the normal temperature aqueous solution passing through will be heated and evaporated.
②The second heat exchanger
The water vapor after the first step of heating will pass through the second heater for further heating. In this step, the particles and impurities in the aqueous solution will be separated.
③Water tank
The water vapor after heating and condensation will enter the water tank. There are two pressure sensors on the water tank. And the water level is automatically adjusted by the equipment.
④Condenser
The condenser will collect the water vapor after high temperature evaporation, and then condense it at low temperature to further remove pollutants in the water.
⑤Heater
The heater mainly uses electric heating or steam heating to further heat the water temperature to the evaporation temperature. Then push the water vapor to the top.
⑥Blower
The blower will push the evaporated water vapor to the dome, and then further condense it to both sides of the shell through the condenser.
⑦Re-circulation tank
The re-circulation tank will easily recollect and circulate the condensed water.
⑧ Booster pump
The booster pump will transfer the condensed water from the recirculation system to the drain pipe of the distiller.
⑨ Distiller drain pipe
The treated distilled water will be discharged through this drain pipe and collected.
Main parts of membrane-based WFI system are:
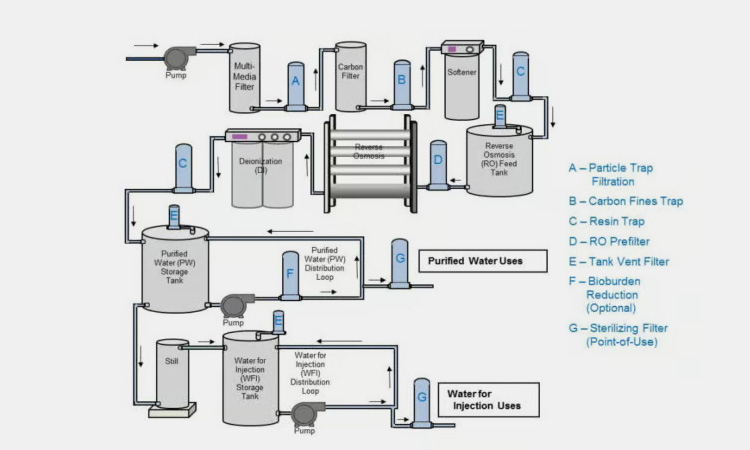
Main parts of membrane-based WFI system-sourced: genspark
Particle trap filtration
The particle trap filtration can filter and remove large particles and contaminants from aqueous solutions.
Carbon fines trap
The carbon fines trap can use the activity of carbon to adsorb various active substances in aqueous solutions.
Resin trap
The resin trap can help remove remaining dissolved salts and minerals in aqueous solutions.
RO prefilter
The RO prefilter can help remove remaining ions in aqueous solutions, such as sodium, potassium, calcium, and magnesium.
Tank vent filter
The tank vent filter can effectively remove large molecular impurities such as bacteria and viruses from aqueous solutions.
Bioburden reduction
The bioburden reduction can improve the odor and taste of water.
Sterilizing filter
The sterilizing filter is the last step in water treatment. It usually uses ultraviolet (UV) light or heat to sterilize the aqueous solution to ensure that the aqueous solution does not contain microorganisms.
8.What Are The WFI Specifications For WFI System?
Before water for injection is put into use, it will undergo strict supervision and testing. The following are the relevant regulations on WFI quality requirements in different regions:
| USP (US Pharmacopiea) | European Pharmacopoeia | Japanese Pharmacopoeia | |
| Images | 
USP (US Pharmacopiea)-sourced: tsoshop |

European Pharmacopoeia-sourced: edqm |

Japanese Pharmacopoeia-sourced: tsoshop |
| Conductivity | < 1.3 μS/cm at 25°C | < 1.1 μS/cm at 20°C | < 2.1 μS/cm at 25°C |
| Bacteria | <10 cfu / 100 ml | <10 cfu / 100 ml | <10 cfu / 100 ml |
| TOC | < 500 ppb | < 0.5 mg/l*** | < 0.5 mg/l |
| Endotoxin | < 0.25 l.U. / ml | < 0.25 l.U. / ml | < 0.25 l.U. / ml |
9.What Is The WFI System Generation Process?
To produce the water for injection is complicated and not easy to carry out. So you need to follow the steps closely without any mistakes. They are:
The WFI generation process of distillation system
The WFI generation process of distillation system-sourced: bram-cor pharmaceutical equipment
Preheating
You need to preheat the heat exchanger. Then water is fed in and then the water temperature is cooled to the pre-set temperature.
Adjusting the temperature
Adjust the amount of water fed in and then keep the WFI temperature stable.
Re-preheating
When entering the second heat exchanger, you need to preheat the second exchanger and re-condense it.
Vapor compression
When the heated water enters the water tank, the pipes of the condenser will be filled with water. The heated water vapor will evaporate further and then occupy the dome. The distiller will compress the high-temperature water vapor to both sides of the condenser.
Re-circulation
The condensed water vapor will be reabsorbed and then passed through the last heat exchanger, then condensed, and finally processed into qualified and qualified high-quality sterile water.
The WFI generation process of membrane system
The WFI generation process of membrane system-sourced: Syntegon
Pre-treatment
In the first step, you can use sand or cartridge filters to remove large particles and suspended solids from the water.
Reverse Osmosis (RO)
In the second step, the filtered water is treated with a reverse osmosis membrane to remove dissolved salts and other impurities. The RO system is able to separate the dissolved salts from the water by high pressure.
Electrodeionization (EDI)
By using an electrodeionization (EDI) module, the positive and negative ions in the water are exchanged and discharged.
Carbon Filtration
You can use activated carbon to remove residual organic matter from the treated water and improve the smell and taste of the water.
Sterilization
Finally, you need to treat the water for bacteria and microorganisms, etc., using ultraviolet (UV) light or heat.
10.What Are The Advantages Of Two Main WFI System?
Advantages Of Two Main WFI System-sourced: tsaprocessequipments
The advantages of distillation system are:
- It can effectively purify impurities and toxins in water;
- It has a stable structure and mature development, with a series of supporting facilities and solutions;
- It is recognized and approved by almost all countries;
Compared with distillation, the advantages of membrane system are:
- It is simpler and more efficient than distillation;
- The procedures and steps are not as complicated as distillation;
- The system is simpler and the cost is lower;
- It improves sustainability;
11.What Is Cold WFI?

What Is Cold WFI-sourced: syntegon
Unlike traditional hot distillation, cold WFI refers to water treatment at room temperature rather than hot temperature. Distillation, also known as "hot WFI", mainly converts water into steam at high temperature, and then condenses it into sterile water. Cold WFI refers to softening, dechlorinating, reverse osmosis and electrodeionizing water, and then using low-pressure ultrafiltration to sterilize and sterilize it.
12.How To Manage The Risks of Cold WFI?

How To Manage The Risks of Cold WFI-sourced: Navdeep singh sethi
Without high temperature or pressure, treated water may have the potential for bacterial growth. How to avoid this risk? Cold WFI uses biofilms that may easily breed bacteria in low temperature environments. However, this situation can be avoided.
You can offset it by frequently disinfecting with hot water. You can regularly use hot water to disinfect the RO/CEDI/UF components in the cold WFI system.
13.How To Select WFI System To Produce Best Water for Injection?
When considering which WFI system to use, you can consider the following aspects:
Quality and specifications of water for injection
Quality and specifications of water for injection-sourced: vwr
Generally, the use of thermal distillation is the best way to ensure the quality of your WFI product. Because impurities and toxins in the water will be completely removed after high temperature and condensation. And different countries have different acceptance of different methods. Europe is more accepting of thermal distillation.
Sustainability
Sustainability-sourced: crbgroup
Thermal distillation requires heating and pressurization, which consumes a lot of energy and puts a lot of pressure on the environment. Membrane systems are mainly operated at room temperature and pressure, with a small carbon footprint and strong sustainability.
Existing equipment and systems
The costs of different equipment systems are different. Thermal distillation systems are more complex and energy-intensive, with high costs and more frequent maintenance. Membrane systems are simpler, less energy-intensive, easier to repair and less expensive.
Other factors

Other factors-sourced: withplum
Other factors to consider include operator training, operational complexity, space occupied by different equipment and systems, your budget, and your staff's experience in making clean water. These are all issues you need to consider.
14.Which Type Of WFI System Is Most Sustainable?
Of course, the membrane system is more sustainable. The cold WFI system mainly uses cold treatment, which can help you reduce your carbon footprint. Hot distillation consumes more energy and has more complicated steps and equipment.
Cons of distillation WFI system:
Energy consumption

Energy consumption-sourced: genial
Distillation requires a lot of resources to boil water and then cool it to the appropriate temperature. And it is inefficient, achieving only half of the total energy consumption.
Cons of membrane-based WFI system:
Chinese regulations do not allow
China still stipulates that water for injection must be made by hot distillation, and it cannot be replaced by other methods. Therefore, if you use a membrane-based WFI system, your product will not be put into the Chinese market.
15.What Are The Differences Between PW And WFI?
PW refers to purified water. The difference between it and WFI is:
| PW | WFI | |
| Full name | Purified water | Water for injection |
| Images | 
Purified water-sourced: senpharma |

Water for injection-sourced: greyhoundchrom |
| Use | Used for oral or external use to clean wounds; | Used for parenteral injection;
|
| Strictness | Only minerals, organic matter and microorganisms need to be removed; | The specifications of WFI are stricter than PW;
And its quality specifications are strictly monitored; |
| Standard criteria | FDA and cGMP; | USP, PhEur or EP), JP; |
| Equipment requirements | All equipment used to produce water must be carefully inspected and verified and comply with relevant regulations; | All equipment used to produce water must be carefully inspected and verified and comply with relevant regulations;
Installed correctly and operated legally; Strictly monitor subsequent operational compliance and quality safety; |
| Treatment methods | Reverse osmosis (RO), ion exchange (IX) and distillation; | Two-pass RO, ion exchange and ultrafiltration (UF);
Hot distillation; |
16.What Are The WFI Testing And Sampling Process?
After the production of WFI is completed, there will be a mature detection mechanism to monitor whether there are any problems with the water quality. The WFI testing and sampling process is:

What Are The WFI Testing And Sampling Process-sourced: honeymanlaboratories
Routine sampling
You need to perform routine sampling of the aqueous solution in the entire water system, and do not miss any part;
Collect samples
Collect and label the various sampled aqueous solutions;
Be careful not to contaminate the samples due to improper techniques, hygiene habits and poor sterilization methods;
Perform tests
Test the collected samples in batches for TOC, conductivity, bacteria and endotoxin levels, etc.;
And record the relevant data for later analysis and comparison;
Compare and analyze
Compare and analyze the test results with the relevant regulations and standards;
So as to trace the source and analyze which specific part has problems;
Form a report
Form a relevant report and issue a certificate;
17.What Is The EP For WFI System?

European Pharmacopoeia-sourced: edqm
EP stands for the European Pharmacopoeia. It is the international standard for water for injection recognized in Europe. EP mainly stipulates various parameters and control standards such as organic carbon, nitrate, chloride, microbial limits and oxidizable substances in water for injection. The following is a detailed introduction to its thresholds.
| Items | EP Water Specification |
| Full name | European Pharmacopoeia |
| Nitrates | 0.2 ppm max |
| Acidity or Alkalinity | Acidity-No blue colour develops |
| Chlorides | No change occurs |
| Sulfates | No change occurs |
| Ammonium | NMT 0.2 ppm |
| Heavy Metals | 0.1 ppm max |
| Residue on Evaporation | NMT 0.001% |
| Microbial Contamination (TAMC) | ≤102CFUmL (200 CFU/mL) |
| Microbiological Monitoring | ≤100 CFU/mL |
Conclusion:
The quality of water for injection cannot be compromised. It is related to human health. The WFI system is the best solution to provide you with high-quality sterile water. Through this complete FAQ guide of WFI system, you may know how to control and secure your quality of WFI. For more you want to know, please contact AIPAK Engineering now!
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours

