Non-PVC IV Bag Production Line: The Complete FAQ Guide In 2025
The non-PVC IV bag production line is a state-of-the-art and up-to-date innovation in the pharmaceutical and healthcare industry. It is equipped with the latest computerized technology with which it automatically performs different functions like feeding, printing, bag forming, and many more.
Picture Courtesy: B. Braun
Before installing this machine, first gather knowledge about the non-PVC IV bag production line by reading through this blog. We have compiled this informative FAQ guide to add to your understanding of the non-PVC IV bag production line.
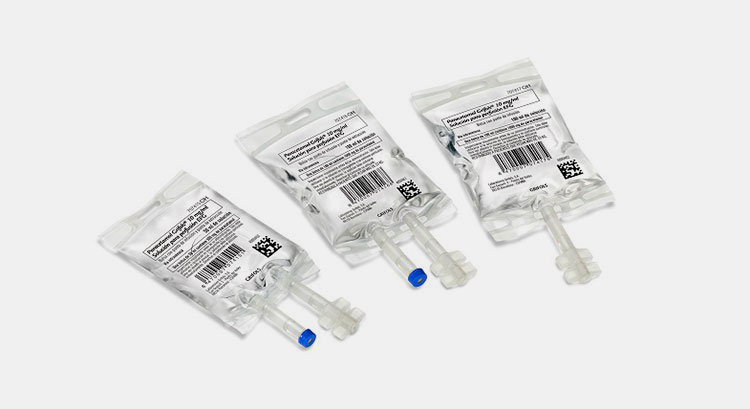
1.What is a Non-PVC IV Bag Production Line?

Non-PVC IV Bag Production Line- Picture Courtesy: GMPMAX
A non-PVC IV bag production line is defined as different machines and processes that perform the job of creating non-PVC IV bags without using PVC materials. This production line has integration of different equipment that are advanced technologically and is fully automated. It uses other plastic materials like polyethylene and polypropylene to manufacture non-PVC IV bags.
Traditionally PVC was used for the creation of IV bags but with the environmental and safety concerns, manufacturers have shunned the practice of using PVC for manufacturing IV bags and now, they are using non-PVC materials for making non-PVC IV bags.
2.What are the Pros of a Non-PVC IV Bag Production Line?
This production line is automated and equipped with modern technology, thus bringing lots of benefits to the production plants. The addition of this production line is also advantageous for the health of patients and the environment. Some of the pros of this production line are listed below for your information.
Enhanced Patient Safety
Enhanced Patient Safety- Picture Courtesy: Peak Health Group
A non-PVC IV bag production line plays a vital role in patient safety because it manufactures IV bags from non-PVC materials. These materials do not have phthalates that are present in PVC material and cause adverse reactions in patient and compromise their health. Moreover, non-PVC materials do not interact with drugs, so they have better drug compatibility.
Eco-Friendliness
Eco-Friendliness of non-PVC IV bag production line- Picture Courtesy: Dechra
A non-PVC IV bag production line produces non-PVC IV bags that are easily recycled and generate decreased waste. This production line is eco-friendly and sustainable because non-PVC IV bags produced by this production line have lower environmental effects, as they release fewer harmful substances into the environment.
Increased Sterility
Increased Sterility- Picture Courtesy: sportgranada.com
A non-PVC IV bag production line has increased sterility because it uses advanced methods like gamma rays for sterilizing the bags. These methods may deteriorate PVC materials but non-PVC materials. This feature boosts sterilizing efficiency and proficiency.
Production Efficiency
Production Efficiency of Non-PVC IV Bag Production Line- Picture Courtesy: B. Braun Medical Inc.
A non-PVC IV bag production line has up-to-date computational and mechanical technology with which it effectively carries out its different jobs. These automated technologies improve production speed and accuracy, leading to fewer error rates. High speed and accuracy generate high profits for pharmaceutical and healthcare companies.
Cost-Effectiveness
Cost-Effectiveness of Non-PVC IV Bag Production Line- Picture Courtesy: Ryvis Pharma
Even though the initial purchase expense of a non-PVC IV bag production line is quite high, in the long run, it earns its investment cost with interest. This is because this production line can fulfill higher production demands due to higher operational efficiency. This improves its cost-effectiveness.
3.What are the Types of Non-PVC IV Bag Production Line?
There are different types of non-PVC IV bag production lines available in the market, differing in their parts and functioning. These are detailed below:
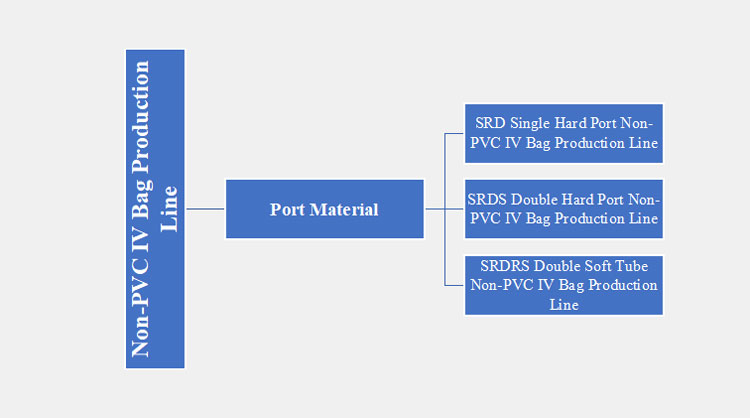
Port Material
There are three main types of non-PVC IV bag production lines based on the port materials. These types are discussed below:
SRD Single Hard Port Non-PVC IV Bag Production Line

AIPAK Engineering Single Hard Port Non-PVC IV Bag Production Line
Single hard port non-PVC IV bag production line places a single hard or rigid port in the non-PVC IV bags. This port is then connected to a medical tube for administration of the IV fluids. It is a completely independent solution that has higher efficiency and increased output rate. It produces 1200-6000 ml single port non-PVC IV bags.
SRDS Double Hard Port Non-PVC IV Bag Production Line

AIPAK Engineering Double Hard Port Non-PVC IV Bag Production Line
It is the more advanced machine and has a larger footprint than its single hard port non-PVC bags production line counterpart. It inserts double rigid ports in the non-PVC IV bags. It performs two pre-heating, two heat seal welding, and one cool welding to put the double hose into the non-PVC IV bags. It can produce non-PVC IV bags with a volume of 1000-5000 ml.
SRDRS Double Soft Tube Non-PVC IV Bag Production Line

AIPAK Engineering Double Soft Port Non-PVC IV Bag Production Line
This kind of non-PVC IV bag production line generates non-PVC IV bags that are equipped with two soft ports. The two flexible ports in the non-PVC IV bags have ease of drug administration. Double soft tube non-PVC IV bag production line creates non-PVC IV bags that reduce the chances of needle injuries and are easier to handle. This production line has a smaller footprint and is made with stainless steel which is cleaning convenience.
4.How Does the Non-PVC IV Bag Production Line Work?
A non-PVC IV bag production line is a huge production line, carrying out different working steps efficiently and effectively. These steps occur with complete synchronization to achieve seamless processing. These steps are detailed below:
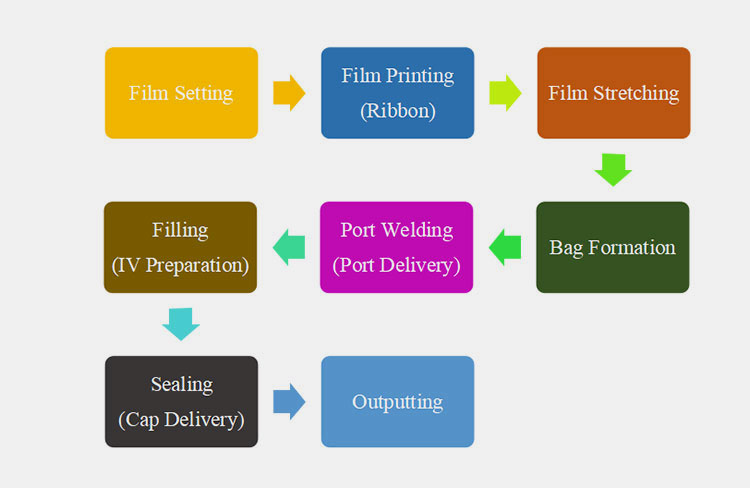
The process steps of the Non-PVC IV Bag Production Line
Film Setting
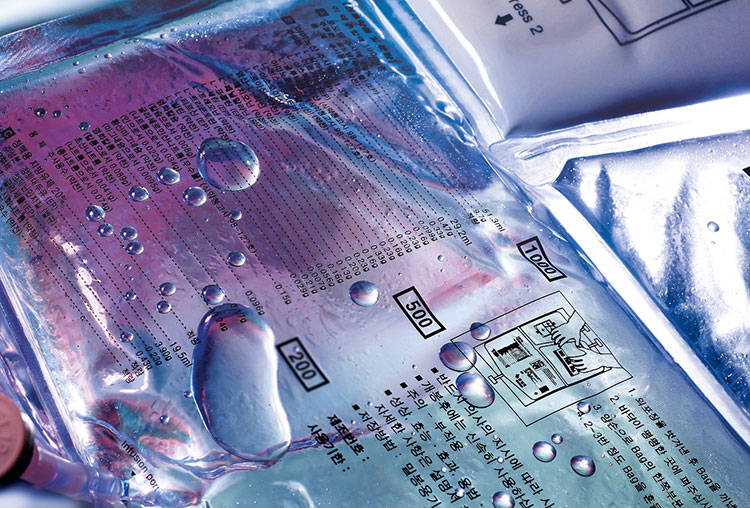
Non-PVC IV Bag Film- Picture Courtesy: CPHI Online
The film material is first adjusted in the machine system depending on the required measurements. The setting involves various channels including rollers, heat sealers, and other related parts to provide smooth and proper movement of the process.
Film Printing

Film Printing
At this station, various information facts like IV bag ingredients, usage, expiry date, bar codes, etc., are printed on the ribbon film using inkjet or thermal transfer printer.
Film Stretching

Film Stretching
First, the film for bag formation is put on the unwinding disk. This film is opened and stretched by the film's open plate. After opening and stretching, the film is doubled and is moved to the printing station.
Bag Formation
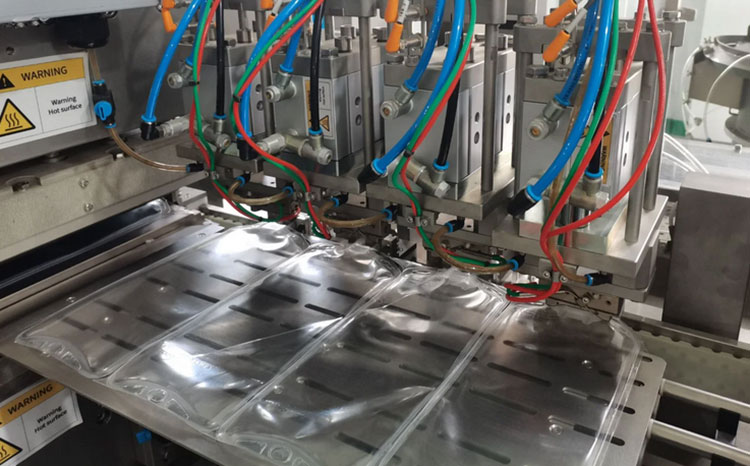
Bag Formation- Picture Courtesy: ivenpharmatech
This station is equipped with a bilateral molding structure that moves up and down to create bag molds from the film. The film is heated to 140°C and above to create the bag. The cooling plate immediately lowers the temperature of the film after its bag creation.
Port Welding

1st and 2nd Port Welding- Picture Courtesy: ivenpharmatech
Depending upon the type of non-PVC IV bag production line, the 1st and 2nd ports are inserted in the non-PVC IV bags. These ports or spouts are placed in the non-PVC IV bags by the heat-sealing methods. These ports are welded in the bags to form a sterile and contamination-free closure.
Filling

Filling of non-PVC IV bags- Picture Courtesy: nsportgranada.com
After port welding, the next step is bag filling in which the aseptic solution is filled by the mass flowmeter measurement system in sterile conditions to prevent contamination of the IV solution.
The flow meter is an accurate system that precisely dispenses IV solutions in the bags to prevent inaccurate dosing.
Sealing

Non-PVC IV Bag Sealing
Sealing is carried out by cylinder driving. The open side of the IV bag is closed using cap delivery method that work by picks and twist on narrow neck followed.
Outputting

Outputting- Picture Courtesy: IVEN Pharma
The bags are then removed from the production line by the conveyor belt and are moved to down-the-line packaging and inspection systems.
5.What Solutions are Filled by Non-PVC IV Bag Production Line?
The non-PVC IV bag production line is capable of filling a miscellaneous range of IV solutions, fulfilling diverse medical needs. Some of the common IV solutions filled by this production line are written for your understanding.
| Saline Solution
Various concentrations of sodium chloride are filled in IV bags. In emergencies, these solutions are used for solving hydration problems. It is also used in medical dilution. |
Saline Solution-Picture Courtesy: Acre Pharmacy |
| Dextrose Solution
These solutions contain different concentrations of dextrose and are injected in patients who have hypoglycemia, insulin shock, and dehydration. |

Dextrose Solution- Picture Courtesy: B. Braun Medical Inc. |
| Amino Acid Solution
It is used for treating nutrition disorders in patients who cannot consume food by mouth. It includes several amino acids like pleamine and tropoamine. |
Amino Acid Solution- Picture Courtesy: B. Braun Medical Inc. |
| Electrolyte Solution
These IV solutions contain important electrolytes like potassium, calcium, and magnesium. It is used for preventing electrolyte imbalance in patients with dehydration, kidney disease, and metabolic disorders. |
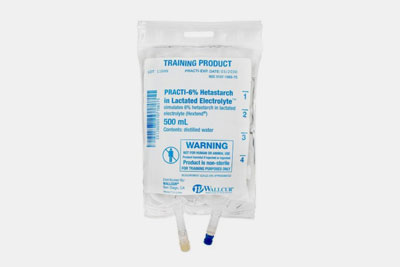
Electrolyte Solution- Picture Courtesy: Anatomy Warehouse. |
| Antibiotics Solution
For treating bacterial and other microbial infections, antibiotics solutions containing a diverse range of antibiotics and other medications are used. These solutions are directly injected into patients. |
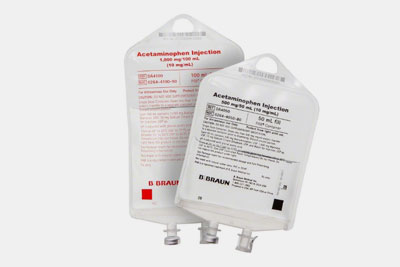
Antibiotics Solution- Picture Courtesy: - Picture Courtesy: B. Braun Medical Inc. |
| Chemotherapy Solution
Non-PVC IV bags are also used for storing aggressive chemotherapy agents. These IV solutions in bags have stability and inertness, increasing the effectiveness of chemotherapy. |

Chemotherapy Solution- Picture Courtesy: Medical Expo |
| Biologics
Different types of biological products like vaccines, hormones, and sera are also filled by the non-PVC IV bag production line. |
Biologics- Picture Courtesy: Technoflex |
6.What are the main Non-PVC IV Bag Production Line units?
The main units of non-PVC IV bag production line are described below:
| Belt Conveyor
|
The Non PVC IV bags belt conveyor is used to transfer bags during the performance. This is a simple and easy-to-use system that helps in lowering labor requirements. Additionally, the conveyor belt can be customized and regulated depending on your production requirements. For example, the speed, dimensions, and other parameters. |  |
| Leakage Inspection Machine
|
To ensure that Non PVC IV bags are manufactured as recommended by international quality standards, a leakage inspection machine is integrated in the production line which is helpful in assessing preliminary sealing quality.
The machine adopts high-pressure assessment methods thus, it is easy to find out the presence of non-compliance and leakage. It is capable of regulating the pressure intensity and easy to adjust depending on various bag sizes. |
|
| Bag Loading Machine
|
This is an easy and labor-saving solution that pushes the filled bags into the sterilization machine for the decontamination process. The loading of the bags is done by machine itself into the sterilizer. |  |
| Bag Unloading Machine
|
The machine is following an auto-lifting procedure to unload the Non-PVC IV bags from the sterilization cart. The machine is able to push the bags out of the cart and the operator is responsible for unloading those bags and assembling them onto a tray for further steps. |  |
| Orbit and Turntable System
|
This is a crucial approach that is required to facilitate the smooth workflow during non IV PVC bag production and assess flexibility during the manufacturing and packaging of the production process. |  |
| Autoclave
|
For sterile non- PVC IV bags, an autoclave is integrated into the production line which ensures ultra cleanliness and safety and provides bags that are contaminant free by induction at a high temperature of around 180 degrees centigrade. |  |
| Trolley
|
The trolley is required that plays a significant role in facilitating fast movement of non PVC IV bags that requires minimal handling. |  |
| Light Inspection Machine
|
This unit is required for deep examination of bag that must be defect free. It involves physical appearance, color of solution inside, presence of foreign particles etc., |  |
| Pillow Type Packaging Machine
|
The machine is integrated to form, fill, and seal a bag which is mostly used for secondary packaging or external packaging of the non PVC IV bags,. An automatic machine which is broadly preferred in various other production fields also due to its excellent packaging output. |  |
| Carton Machine
|
For transportation and safe delivery of non PVC IV bags, its packaging is done by carton machine which utilizes flat cartons and capable of folding, feeding, and enclosing to secure your product during shipment. |  |
| Laminar Flow
|
Without a laminar flow setup, a clean and hygienic working condition is considered incomplete. This unit offers a well-controlled and regulated air flow to maintain extra cleanliness or contamination free facility | 
Picture Courtesy: Sentry Air Flow |
7.What Type of Materials are Processed by the Non-PVC IV Bag Production Line?
The non-PVC IV bag production line processes different materials that are substitutes to the traditional PVC. Manufacturers have adopted using non-PVC materials because of health and safety concerns. Here is the list of materials processed by the non-PVC IV bag production line.
Polyethylene (PE)
PE IV Bag- Picture Courtesy: Mountainside Medical Equipment
This material is commonly used for manufacturing IV bags because it has higher elasticity and greater chemical resistance. It is compatible with most of the IV solutions. Furthermore, it has decreased toxicity. PE is used in combination with other materials to boost barrier protection and robustness of IV bags.
Polypropylene (PP)
PP IV Bag- Picture Courtesy: ICU Medical
It is one of the most frequently used materials for creating IV bags. It has superior chemical and barrier protection. It is heat-stable so, it is best for heated sterilization techniques like steam sterilization or autoclaving.
Copolyesters
Copolyester IV Bag- Picture Courtesy: Nasco Healthcare
Due to their high transparency, tensile strength, and elasticity, copolyester is utilized for making IV bags. These materials offer superior impermeability to the oxygen, carbon dioxide, and water content, so it is used for storing sensitive IV solutions.
Ethylene Vinyl Acetate (EVA)
EVA IV Bag- Picture Courtesy: Fresenius Kabi
It is a safer substitute for housing IV solutions because does not need a plasticizer during its manufacturing. It has cold-temperature plasticity and excellent transparency. It has resistance against cracking and UV radiation, so it is preferred by many manufacturers.
8.What are the Sterilization Methods in Non-PVC IV Bag Production Line?
Sterilization is an important step in the non-PVC IV bag production line. It inhibits the production of microbes in the non-PVC IV bags and increases product safety. Here is a list of different sterilization methods performed in the non-PVC IV bag production line:
Gamma Radiation

Gamma Sterilization- Picture Courtesy: Assembly Magazine
It is one of the most common methods for sterilization of IV solution bags. In this method, ionization radiations from cobalt-60 are passed through non-PVC IV bags. These ionization radiations change and destabilize the microbial DNA and destroy bacteria, viruses, and other microbial agents.
Electronic Beam Sterilization

E-beam Sterilization- Picture Courtesy: System BD
In this method, a high-energy electronic beam is subjected to the non-PVC IV bag. It also destroys the DNA of microbes, effectually killing them. It is fast and does not need radioactive substances.
Autoclave Sterilization

Autoclave Sterilization- Picture Courtesy: Fedegari
It is also called steam sterilization. In this sterilization technique, high-pressure steam is used to sterilize the surfaces and inner contents of the non-PVC IV bag. It degrades microbes by altering and denaturing their protein structure. It is used both before and after filling IV solution bags.
Chemical Sterilization

Chemical Sterilization- Picture Courtesy: Business Wire
In this method, gaseous chemicals like ethylene oxide are passed through the non-PVC IV bags. It is harmful to microbial DNA and other molecular structures like proteins. This sterilization technique is mostly used for heat-sensitive materials.
9.What Regulatory Certification Should Non-PVC IV Bag Production Line Have?
A non-PVC IV bag production line must comply with strict regulatory protocols to ascertain the safety, effectiveness, and quality of the end products. Adherence to various regulatory obligations is essential in acquiring different regulatory certifications. Below are some fundamental regulatory certifications that the non-PVC IV bag production line must have:
ISO 13485

ISO 13485-Picture Courtesy: RegDesk
This ISO certification regulates the manufacturing, production, marketing, and retailing of medical instrumentation such as non-PVC IV bags. A non-PVC IV bag production line must comply with ISO 13485 obligations to showcase its commitment to creating high-quality IV bags and to uphold the safety and effectiveness of IV solutions.
Good Manufacturing Practice

Good Manufacturing Practice- Picture Courtesy: ERIKs
It should be the objective of the non-PVC IV bag production line to acquire GMP certification. Having this certification shows non-PVC IV bag production line follow safe, clean, sterile, and hygienic practices while manufacturing the non-PVC IV bags.
CE Marking

CE Marking
This certification is mandatory for the non-PVC IV bag production line operating and manufacturing non-PVC IV bags in the European Union. This production line must pass conformity test protocols and fulfil safety and quality requisites to acquire CE certification.
FDA

FDA
The Non-PVC IV bag production line working in the United States must be listed with USA Food and Drug Admiration. Complying with FDA guidelines ascertains that manufacturing processes are dependable and regulated and create good-quality reliable products.
10.What Quality Control Tests are Implemented in the Non-PVC IV Bag Production Line?

Quality Control Tests of non-PVC IV Bag Production Line-Picture Courtesy: Medline
Quality control is an important part of the non-PVC IV bag production line. It ensures that IV bags are manufactured by fulfilling the highest stringent quality protocols. We are detailing some important quality control tests like:
| Visual Inspection Test | In this test, different visual artifacts like clarity, printing inaccuracies, color differences, presence of foreign particles, and seal integrity are checked visually by the operator. It also identifies the presence of caps and ports in the IV solution bags. |
| Volume Precision Test | This test determines the accuracy of the volume by weighing the IV bags. It checks that the volume of the IV bag is according to the required level. |
| Seal Integrity Test | Different tests like dye penetration tests and vacuum decay tests are conducted to check the seal integrity like its robustness and leaking. |
| Leak Test | It determines that the bag does not leak after it is filled and sealed. Different pressure difference tests and dye immersion tests are performed to check the leaking of IV bags. |
| Sterility Test | This test is performed to check the presence of microbes and sterility of the inner bag contents. The contents of the IV bags are cultured to identify microbial contamination. Afterwards, a sterility assurance level test is conducted. |
| Tensile Strength Test | This test checks the strength and elasticity of the non-PVC IV bags. It is performed using a tensometer that pulls the IV bags till their breakage point. The force needed to break the bag and material elongation at breakage give an idea of the tensile strength and elasticity of the IV bags. |
| Material Compatibility Test | This test ascertains the compatibility of the materials and the IV solutions. It ensures that there is no reactivity between bag materials and the IV solution and that the composition of the IV solution does not change upon storing the bag for a longer period. |
| Pressure Resistance Test | This test is conducted to inspect the ability of non-PVC IV bags to be resistant to extreme pressure changes during transportation. The bags are exposed to high-pressure ranges and their breakage and elongation are then measured. |
11.What are the Challenges and Solutions of Non-PVC IV Bag Production Line?
The installation of a non-PVC IV bag production line is filled with plenty of challenges. However, for each challenge, there is a solution. We are explaining the challenges and solutions of the non-PVC IV bag production line below:
Material Processing

Material Processing- Picture Courtesy: McGuff
Materials like PP, PE, and EVA have diverse handling needs when compared with traditional PVC. There is a difference in the melting point and sealing temperature of these materials.
Solution
This challenge is resolved by installing a non-PVC IV bag production line, capable of processing these materials. Also, testing and adjusting melting and sealing parameters should resolve the problem of material processing.
Film Material Cost

Film Material Cost- Picture Courtesy: EuroPlas
The non-PVC materials are more costly than PVC materials, this increases the operational cost.
Solution
Higher material costs can be reduced by recycling non-PVC materials. Also, considering patient safety and eco-friendliness of non-PVC materials can justify higher purchase costs of non-PVC materials.
Seal Integrity
Seal Process of IV Bags- Picture Courtesy: CPHI Online
A durable and strong seal is essential for the safety of IV solutions. Sealing techniques and conditions of non-PVC materials are difficult to optimize.
Solution
This problem is resolved by investing in advanced ultrasonic methods and laser welding techniques. This advanced sealing technology can improve the seal steadiness and toughness of non-PVC IV bags.
Compatibility with the Filling Solution

Compatibility with Fill Solution- Picture Courtesy: Nasco Healthcare
Some IV fluid solutions are incompatible with the non-PVC materials, possibly causing the degradation of IV bags and a change in the composition of the solution.
Solution
Before marketing and retailing the IV bags, first conduct thorough compatibility testing for IV solution and non-PVC materials. This testing can aid in choosing of right IV bag materials.
Precision of Filling Solutions

Precision of Filling Solutions- Picture Courtesy: Technoflex
Accurate volume control is essential for the correct dosage. Precision in the fill volume is changed by the inconsistencies in the thickness and flexibility of IV bags.
Solution
Investing in high-precision filling devices like flowmeter solves this challenge. These devices are adjusted to the variations in the IV bag materials. This allows you to achieve accurate dosing.
Conclusion
The non-PVC IV bag production line processes non-PVC materials that present the best alternative to out-of-date PVC. It is a need of the pharmaceutical and healthcare industry as it offers state-of-the-art IV bag manufacturing, filling, and sealing. This production line has experienced high growth in the latest era because of its safety, sustainability, accuracy, and cost-effectiveness. It is integrated into the IV bag production plant because of its quality control and hygienic handling. Now it is time to conclude this FAQ guide, for further queries about the non-PVC IV bag production line, please contact AIPAK Engineering customer care.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours


